Antimania-Like Effect of Panax ginseng Regulating the Glutamatergic Neurotransmission in REM-Sleep Deprivation Rats
- PMID: 33123570
- PMCID: PMC7586145
- DOI: 10.1155/2020/3636874
Antimania-Like Effect of Panax ginseng Regulating the Glutamatergic Neurotransmission in REM-Sleep Deprivation Rats
Abstract
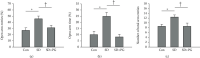
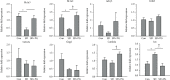
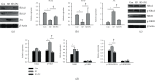
Previous studies have shown the therapeutic properties of ginseng and ginsenosides on hyperactive and impulsive behaviors in several psychiatric diseases. Herein, we investigated the effect of Panax ginseng Meyer (PG) on hyperactive/impulsive behaviors in a manic-like animal model, sleep deprivation (SD) rats. Male rats were sleep-deprived for 48 h, and PG (200 mg/kg) was administered for 4 days, from 2 days prior to the start of SD to the end date of SD. The elevated plus maze (EPM) test showed that PG alleviated the increased frequency of entries into and spent time within open arms by SD. In order to investigate the molecular mechanism on this effect of PG, we assessed differentially expressed genes (DEGs) in the prefrontal cortex of PG-treated SD rats using RNA sequencing (RNA-seq) and performed gene-enrichment analysis for DEGs. The gene-enrichment analysis showed that PG most prominently affected the glutamatergic synapse pathway. Among the glutamatergic synapse pathway genes, particularly, PG enhanced the expressions of glutamate transporter Slc1a3 and Slc1a2 reduced in SD rats. Moreover, we found that PG could inhibit the SD-induced phosphorylation of the NR2A subunit of the NMDA receptor. These results suggested that PG might have a therapeutic effect against the manic-like behaviors, regulating the glutamatergic neurotransmission.
Copyright © 2020 Kang Hyun Leem et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

