Impact of tumor heterogeneity and microenvironment in identifying neoantigens in a patient with ovarian cancer
- PMID: 33123756
- PMCID: PMC8053669
- DOI: 10.1007/s00262-020-02764-9
Impact of tumor heterogeneity and microenvironment in identifying neoantigens in a patient with ovarian cancer
Abstract
Identification of neoepitopes as tumor-specific targets remains challenging, especially for cancers with low mutational burden, such as ovarian cancer. To identify mutated human leukocyte antigen (HLA) ligands as potential targets for immunotherapy in ovarian cancer, we combined mass spectrometry analysis of the major histocompatibility complex (MHC) class I peptidomes of ovarian cancer cells with parallel sequencing of whole exome and RNA in a patient with high-grade serous ovarian cancer. Four of six predicted mutated epitopes capable of binding to HLA-A*02:01 induced peptide-specific T cell responses in blood from healthy donors. In contrast, all six peptides failed to induce autologous peptide-specific response by T cells in peripheral blood or tumor-infiltrating lymphocytes from ascites of the patient. Surprisingly, T cell responses against a low-affinity p53-mutant Y220C epitope were consistently detected in the patient with either unprimed or in vitro peptide-stimulated T cells even though the patient's primary tumor did not bear this mutation. Our results demonstrated that tumor heterogeneity and distinct immune microenvironments within a patient should be taken into consideration for identification of immunogenic neoantigens. T cell responses to a driver gene-derived p53 Y220C mutation in ovarian cancer warrant further study.
Keywords: Immunotherapy; Mass spectrometry; Neoepitopes; Ovarian cancer; Parallel sequencing; T cell response.
Conflict of interest statement
David Scheinberg is on the advisory board of, has received consulting fees from, and/or has equity in Progenics Pharmaceuticals, Sellas, KLUS, Iovance Biotherapeutics, Inc., Pfizer, Actinium Pharmaceuticals, Inc., OncoPep, and Eureka Therapeutics. Tao Dao has equity in Eureka Therapeutics. Dr O'Cearbhaill's institution receives funding for clinical research that she is leading from Celgene/Juno, Tesaro/GSK, Ludwig Cancer Institute, Abbvie, Regeneron, TCR2 Therapeutics, Atara Biotherapeutics, MarkerTherapeutics, Syndax Pharmaceuticals, Genmab Therapeutics, Sellas Therapeutics, Genentech, Kite Pharma, and the Gynecologic Oncology Foundation. Dr O’Cearbhaill has served on a once-off advisory board for Regeneron, Genmab Therapeutics, and GSK. All other authors declare no conflict or competing interests.
Figures

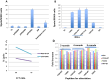

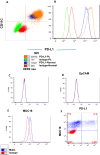

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

