Diagnostic accuracy of anti-phospholipase A2 receptor (PLA2R) antibodies in idiopathic membranous nephropathy: an Italian experience
- PMID: 33123964
- PMCID: PMC8036194
- DOI: 10.1007/s40620-020-00888-w
Diagnostic accuracy of anti-phospholipase A2 receptor (PLA2R) antibodies in idiopathic membranous nephropathy: an Italian experience
Abstract
Background: Autoantibodies against-phospholipase A2 receptor (PLA2R) are specific markers of idiopathic membranous nephropathy (iMN). Enzyme-linked immunosorbent assay (ELISA) is becoming the preferred method in many laboratories for the determination of anti-PLA2R antibodies, because it provides quantitative results, and is not prone to subjective interpretation, as is the case with indirect immunofluorescence assay.
Methods: The purpose of our study was to determine the diagnostic performance of serum PLA2R antibodies detected by commercially available ELISA in a large Italian multicenter cohort of patients with biopsy-proven iMN and in patients with other renal diseases, with special focus on evaluating the optimal cut-off value to discriminate positive and negative results. A total of 495 consecutive patients were recruited. Renal biopsies were performed in all patients, and blood samples were taken before the initiation of immunosuppressive treatment.
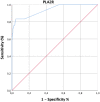
Results: According to the clinical diagnosis and to kidney biopsy, 126 patients were diagnosed with iMN and 369 had other non-membranous nephropathies. Anti-PLA2R autoantibodies were detected using a commercial anti-PLA2R ELISA. At a cut-off value of 20 relative units (RU)/ml indicated by the manufacturer for positive classification, sensitivity was 61.1% and specificity 99.7%. At a cut-off value of 14 RU/ml indicated by the manufacturer for borderline results, sensitivity was 63.5% and specificity remained the same (99.7%). At a cut-off of 2.7 RU/ml, selected as the optimal cut-off on the basis of ROC curve analysis, sensitivity was 83.3% and specificity 95.1%. The best overall efficiency of the test was observed at 2.7 RU/ml; however, the highest positive likelihood ratio and diagnostic odds ratio were achieved at 14 RU/ml. A cut-off threshold higher than 14 RU/ml or lower than 2.7 RU/ml entailed worse test performance.
Conclusion: Depending on the clinical use (early diagnosis or as a support to confirm clinical diagnosis), nephrologists may take advantage of this evidence by choosing the most convenient cut-off. However, renal biopsy remains mandatory for the definitive diagnosis of iMN and for the assessment of disease severity.
Keywords: Anti-phospholipase A2 receptor (PLA2R) autoantibodies; Cut-off value; Enzyme-linked immunosorbent assay (ELISA); Membranous nephropathy.
Conflict of interest statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources