Validation and Implementation of a Highly Sensitive and Efficient Newborn Screening Assay for Mucopolysaccharidosis Type II
- PMID: 33124617
- PMCID: PMC7711921
- DOI: 10.3390/ijns6040079
Validation and Implementation of a Highly Sensitive and Efficient Newborn Screening Assay for Mucopolysaccharidosis Type II
Abstract
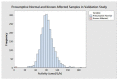
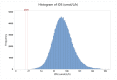
Mucopolysaccharidosis Type II (MPS II), also known as Hunter syndrome, is a lysosomal storage disorder (LSD) caused by a deficiency of the lysosomal enzyme iduronate-2-sulfatase (IDS). MPS II satisfies all criteria defined by the Advisory Committee on Heritable Disorders in Newborns and Children (ACHDNC) for inclusion in the Recommended Uniform Screening Panel (RUSP) for newborn screening, apart from the fact that only minimal prospective population screening data are available. This report details the analytical validation, clinical validation, and implementation of a fluorometric assay for measurement of IDS activity in newborn dried blood spot (DBS) specimens at the Missouri State Public Health Laboratory (MSPHL). The assay is performed in a microwell plate format requiring approximately 15 min of hands-on time per plate and an incubation time of two hours. The analytical validation of this assay included linearity, analytical sensitivity, precision, and carry-over testing. Clinical validation was completed using more than 5000 deidentified presumptive normal newborn DBS specimens as well as seven specimens from patients known to be affected with MPS II. Following validation, MSPHL began prospective screening using the IDS assay on 1 November 2018. In the first 18 months of screening (to 30 June 2020), 146,954 specimens were prospectively screened using the method. Two newborns were identified with severe Hunter syndrome and the assay had a presumptive positive rate of 0.022%.
Keywords: Hunter syndrome; analytical validation; clinical validation; mucopolysaccharidosis II; newborn screening.
Conflict of interest statement
J.W. has stocks or stock options in Baebies, Inc. All other authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results
Figures

References
-
- Secretary of Health and Human Services Recommendation to Add Pompe Disease to the RUSP. [(accessed on 12 October 2020)]; Available online: https://www.hrsa.gov/sites/default/files/hrsa/advisory-committees/herita....
-
- Secretary of Health and Human Services Recommendation to Add MPS I to the RUSP. [(accessed on 12 October 2020)]; Available online: https://www.hrsa.gov/sites/default/files/hrsa/advisory-committees/herita....
LinkOut - more resources
Full Text Sources

