Agromonas: a rapid disease assay for Pseudomonas syringae growth in agroinfiltrated leaves
- PMID: 33124734
- PMCID: PMC7898395
- DOI: 10.1111/tpj.15056
Agromonas: a rapid disease assay for Pseudomonas syringae growth in agroinfiltrated leaves
Abstract
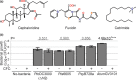
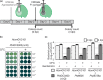
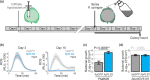
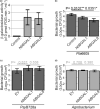
The lengthy process to generate transformed plants is a limitation in current research on the interactions of the model plant pathogen Pseudomonas syringae with plant hosts. Here we present an easy method called agromonas, where we quantify P. syringae growth in agroinfiltrated leaves of Nicotiana benthamiana using a cocktail of antibiotics to select P. syringae on plates. As a proof of concept, we demonstrate that transient expression of PAMP receptors reduces bacterial growth, and that transient depletion of a host immune gene and transient expression of a type-III effector increase P. syringae growth in agromonas assays. We show that we can rapidly achieve structure-function analysis of immune components and test the function of immune hydrolases. The agromonas method is easy, fast and robust for routine disease assays with various Pseudomonas strains without transforming plants or bacteria. The agromonas assay offers a reliable approach for further comprehensive analysis of plant immunity.
Keywords: Agrobacterium; Nicotiana benthamiana; Pseudomonas syringae; disease assay; plant immunity; technical advance.
© 2020 The Authors. The Plant Journal published by Society for Experimental Biology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Abd‐Aziz, N. , Tan, B.C. , Rejab, N.A. , Othman, R.Y. and Khalid, N. (2020) A new plant expression system for producing pharmaceutical proteins. Mol. Biotechnol. 62, 240–251. - PubMed
-
- Bally, J. , Jung, H. , Mortimer, C. , Naim, F. , Philips, J.G. , Hellens, R. , Bombarely, A. , Goodin, M.M. and Waterhouse, P.M. (2018) The rise and rise of Nicotiana benthamiana: a plant for all reasons. Annu. Rev. Phytopathol. 56, 405–426. - PubMed
-
- Buscaill, P. , Chandrasekar, B. , Sanguankiattichai, N. et al (2019) Glycosidase and glycan polymorphism control hydrolytic release of immunogenic flagellin peptides. Science, 364, eaav0748. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

