Inflammation and thrombosis in COVID-19 pathophysiology: proteinase-activated and purinergic receptors as drivers and candidate therapeutic targets
- PMID: 33124941
- PMCID: PMC8238137
- DOI: 10.1152/physrev.00035.2020
Inflammation and thrombosis in COVID-19 pathophysiology: proteinase-activated and purinergic receptors as drivers and candidate therapeutic targets
Abstract
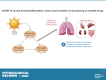
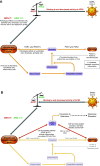
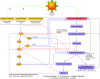
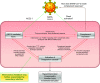
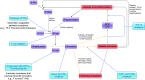
Evolving information has identified disease mechanisms and dysregulation of host biology that might be targeted therapeutically in coronavirus disease 2019 (COVID-19). Thrombosis and coagulopathy, associated with pulmonary injury and inflammation, are emerging clinical features of COVID-19. We present a framework for mechanisms of thrombosis in COVID-19 that initially derive from interaction of SARS-CoV-2 with ACE2, resulting in dysregulation of angiotensin signaling and subsequent inflammation and tissue injury. These responses result in increased signaling by thrombin (proteinase-activated) and purinergic receptors, which promote platelet activation and exert pathological effects on other cell types (e.g., endothelial cells, epithelial cells, and fibroblasts), further enhancing inflammation and injury. Inhibitors of thrombin and purinergic receptors may, thus, have therapeutic effects by blunting platelet-mediated thromboinflammation and dysfunction in other cell types. Such inhibitors include agents (e.g., anti-platelet drugs) approved for other indications, and that could be repurposed to treat, and potentially improve the outcome of, COVID-19 patients. COVID-19, caused by the SARS-CoV-2 virus, drives dysregulation of angiotensin signaling, which, in turn, increases thrombin-mediated and purinergic-mediated activation of platelets and increase in inflammation. This thromboinflammation impacts the lungs and can also have systemic effects. Inhibitors of receptors that drive platelet activation or inhibitors of the coagulation cascade provide opportunities to treat COVID-19 thromboinflammation.
Keywords: GPCR; angiotensin; endothelium; platelets; thrombin.
Conflict of interest statement
P.A.I. is not currently, but within the past 3 years, has served as a consultant or received research support from Merck, Pfizer, and Bristol Myers Squibb. K.S. has no conflicts of interest.
Figures



References
-
- Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med383: 120–128, 2020. doi: 10.1056/NEJMoa2015432. - DOI - PMC - PubMed
-
- Hottz ED, Azevedo-Quintanilha IG, Palhinha L, Teixeira L, Barreto EA, Pão CRR, Righy C, Franco S, Souza TML, Kurtz P, Bozza FA, Bozza PT. Platelet activation and platelet-monocyte aggregates formation trigger tissue factor expression in severe COVID-19 patients. Blood136: 1330–1341, 2020. doi: 10.1182/blood.2020007252. - DOI - PMC - PubMed
-
- Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, Thomas S, Adler NM, Charytan DM, Gasmi B, Hochman JS, Reynolds HR. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine 24: 100434, 2020. doi: 10.1016/j.eclinm.2020.100434. - DOI - PMC - PubMed
-
- Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C, Vander K, Bargfrieder U, Trauner M. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med 173: 350–361, 2020. doi: 10.7326/M20-2566. - DOI - PMC - PubMed
-
- Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MCA, Bouman CCS, Beenen LFM, Kootte RS, Heijmans J, Smits LP, Bonta PI, Es N. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost 18: 1995–2002, 2020. doi: 10.1111/jth.14888. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

