Glycogen synthase kinase 3β in tumorigenesis and oncotherapy (Review)
- PMID: 33125126
- PMCID: PMC7610307
- DOI: 10.3892/or.2020.7817
Glycogen synthase kinase 3β in tumorigenesis and oncotherapy (Review)
Abstract
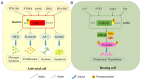
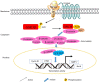
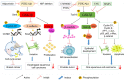
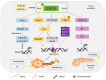
Glycogen synthase kinase 3β (GSK 3β), a multifunctional serine and threonine kinase, plays a critical role in a variety of cellular activities, including signaling transduction, protein and glycogen metabolism, cell proliferation, cell differentiation, and apoptosis. Therefore, aberrant regulation of GSK 3β results in a broad range of human diseases, such as tumors, diabetes, inflammation and neurodegenerative diseases. Accumulating evidence has suggested that GSK 3β is correlated with tumorigenesis and progression. However, GSK 3β is controversial due to its bifacial roles of tumor suppression and activation. In addition, overexpression of GSK 3β is involved in tumor growth, whereas it contributes to the cell sensitivity to chemotherapy. However, the underlying regulatory mechanisms of GSK 3β in tumorigenesis remain obscure and require further in‑depth investigation. In this review, we comprehensively summarize the roles of GSK 3β in tumorigenesis and oncotherapy, and focus on its potentials as an available target in oncotherapy.
Keywords: glycogen synthase kinase 3β; tumorigenesis; oncotherapy; GSK 3β inhibitors.
Figures

Similar articles
-
The mood stabilizing properties of AF3581, a novel potent GSK-3β inhibitor.Biomed Pharmacother. 2020 Aug;128:110249. doi: 10.1016/j.biopha.2020.110249. Epub 2020 May 26. Biomed Pharmacother. 2020. PMID: 32470749
-
The role of glycogen synthase kinase-3β (GSK-3β) in endometrial carcinoma: A carcinogenesis, progression, prognosis, and target therapy marker.Oncotarget. 2016 May 10;7(19):27538-51. doi: 10.18632/oncotarget.8485. Oncotarget. 2016. PMID: 27050373 Free PMC article.
-
Glycogen synthase kinase-3 beta inhibitors as novel cancer treatments and modulators of antitumor immune responses.Cancer Biol Ther. 2019;20(8):1047-1056. doi: 10.1080/15384047.2019.1595283. Epub 2019 Apr 12. Cancer Biol Ther. 2019. PMID: 30975030 Free PMC article. Review.
-
GSK-3β in DNA repair, apoptosis, and resistance of chemotherapy, radiotherapy of cancer.Biochim Biophys Acta Mol Cell Res. 2020 May;1867(5):118659. doi: 10.1016/j.bbamcr.2020.118659. Epub 2020 Jan 21. Biochim Biophys Acta Mol Cell Res. 2020. PMID: 31978503 Review.
-
Glycogen Synthase Kinase-3β as a Putative Therapeutic Target for Bipolar Disorder.Curr Drug Metab. 2018;19(8):663-673. doi: 10.2174/1389200219666171227203737. Curr Drug Metab. 2018. PMID: 29283064 Review.
Cited by
-
NDRG1 and its family members: More than just metastasis suppressor proteins and targets of thiosemicarbazones.J Biol Chem. 2025 Jul;301(7):110230. doi: 10.1016/j.jbc.2025.110230. Epub 2025 May 14. J Biol Chem. 2025. PMID: 40378957 Free PMC article. Review.
-
Chemical Composition, Pharmacological Effects and Clinical Applications of Cinobufacini.Chin J Integr Med. 2024 Apr;30(4):366-378. doi: 10.1007/s11655-024-3708-6. Epub 2024 Jan 12. Chin J Integr Med. 2024. PMID: 38212503 Review.
-
ERMP1 Facilitates The Malignant Characteristics of Colorectal Cancer Cells through Modulating PI3K/AKT/β-Catenin Pathway and Localization of GRP78.Cell J. 2023 Jul 25;25(7):470-482. doi: 10.22074/cellj.2023.1982707.1188. Cell J. 2023. PMID: 37543860 Free PMC article.
-
CircGSK3β mediates PD-L1 transcription through miR-338-3p/PRMT5/H3K4me3 to promote breast cancer cell immune evasion and tumor progression.Cell Death Discov. 2024 Oct 4;10(1):426. doi: 10.1038/s41420-024-02197-8. Cell Death Discov. 2024. PMID: 39366935 Free PMC article.
-
GSK3β palmitoylation mediated by ZDHHC4 promotes tumorigenicity of glioblastoma stem cells in temozolomide-resistant glioblastoma through the EZH2-STAT3 axis.Oncogenesis. 2022 May 23;11(1):28. doi: 10.1038/s41389-022-00402-w. Oncogenesis. 2022. PMID: 35606353 Free PMC article.
References
-
- Thotala DK, Yazlovitskaya EM. GSK3B (glycogen synthase kinase 3 beta) Atlas Genet Cytogenet Oncol Haematol. 2011;15:7–10.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

