Diagnostic validation of a rapid and field-applicable PCR-lateral flow test system for point-of-care detection of cyprinid herpesvirus 3 (CyHV-3)
- PMID: 33125418
- PMCID: PMC7598509
- DOI: 10.1371/journal.pone.0241420
Diagnostic validation of a rapid and field-applicable PCR-lateral flow test system for point-of-care detection of cyprinid herpesvirus 3 (CyHV-3)
Abstract
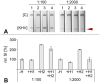
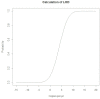
Koi herpesvirus disease (KHVD) is a highly infectious disease leading to outbreaks and mass mortality in captive and free-ranging common carp and koi carp. Outbreaks may result in high morbidity and mortality which can have a severe economic impact along the supply chain. Currently, control and prevention of KHVD relies on avoiding exposure to the virus based on efficient hygiene and biosecurity measures. An early diagnosis of the disease is crucial to prevent its spread and to minimize economic losses. Therefore, an easy-to-handle, sensitive, specific and reliable test prototype for a point-of-care detection of KHV was developed and evaluated in this study. We used a multiplex-endpoint-PCR followed by a specific probe hybridization step. PCR-products/hybridization-products were visualized with a simple and universal lateral flow immunoassay (PCR-LFA). Fifty-four gill tissue samples (KHV-positive n = 33, KHV-negative n = 21) and 46 kidney samples (KHV-positive n = 24, KHV-negative n = 22) were used to determine diagnostic sensitivity and specificity of the PCR-LFA. In addition, the usability of PCR-LFA to detect CyHV-3-DNA in gill swabs taken from 20 perished common carp during a KHVD-outbreak in a commercial carp stock was examined. This assay gave test results within approximately 60 min. It revealed a detection limit of 9 KHV gene copies/μl (95% probability), a diagnostic specificity of 100%, and diagnostic sensitivity of 94.81% if samples were tested in a single test run only. PCR inhibition was noticed when examining gill swab samples without preceding extraction of DNA or sample dilution. Test sensitivity coud be enhanced by examining samples in five replicates. Overall, our PCR-LFA proved to be a specific, easy-to-use and time-saving point-of-care-compatible test for the detection of KHV-DNA. Regarding gill swab samples, further test series using a higher number of clinical samples should be analyzed to confirm the number of replicates and the sample processing necessary to reveal a 100% diagnostic sensitivity.
Conflict of interest statement
Ivo Bertalan is an employee of Milenia Biotech GmbH. There are no patents, products in development or marketed products to declare. This does not alter our adherence to all the PLOS ONE policies on sharing data and materials, as detailed online in the guide for authors.
Figures




Similar articles
-
Laboratory validation of a lateral flow device for the detection of CyHV-3 antigens in gill swabs.J Virol Methods. 2013 Nov;193(2):679-82. doi: 10.1016/j.jviromet.2013.07.034. Epub 2013 Jul 26. J Virol Methods. 2013. PMID: 23896022
-
Initial characteristics of koi herpesvirus and development of a polymerase chain reaction assay to detect the virus in koi, Cyprinus carpio koi.Dis Aquat Organ. 2002 Mar 11;48(2):101-8. doi: 10.3354/dao048101. Dis Aquat Organ. 2002. PMID: 12005231
-
An inexpensive and rapid diagnostic method of Koi Herpesvirus (KHV) infection by loop-mediated isothermal amplification.Virol J. 2005 Oct 17;2:83. doi: 10.1186/1743-422X-2-83. Virol J. 2005. PMID: 16216123 Free PMC article.
-
CyHV-3: the third cyprinid herpesvirus.Dis Aquat Organ. 2013 Jul 22;105(2):163-74. doi: 10.3354/dao02614. Dis Aquat Organ. 2013. PMID: 23872859 Free PMC article. Review.
-
[Koi herpesvirus disease].Uirusu. 2005 Jun;55(1):145-51. doi: 10.2222/jsv.55.145. Uirusu. 2005. PMID: 16308541 Review. Japanese.
Cited by
-
Herpesviruses in Reptiles.Front Vet Sci. 2021 May 5;8:642894. doi: 10.3389/fvets.2021.642894. eCollection 2021. Front Vet Sci. 2021. PMID: 34026888 Free PMC article. Review.
-
Comparison of pre-labelled primers and nucleotides as DNA labelling method for lateral flow detection of Legionella pneumophila amplicons.Sci Rep. 2024 Feb 29;14(1):5018. doi: 10.1038/s41598-024-55703-4. Sci Rep. 2024. PMID: 38424185 Free PMC article.
References
-
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the sustainable development goals. Rome. Licence: CC BY-NC-SA 3.0 IGO. [Cited 2020 March 15]. http://www.fao.org/3/i9540en/i9540en.pdf
-
- Haenen OLM, Way K, Bergmann SM, Ariel E. The emergence of koi herpesvirus and its significance to European aquaculture. Bull Eur Ass Fish Pathol. 2004;24: 293–307. [Cited 2020 March 15]. Available from: https://edepot.wur.nl/198497
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

