Microvascular Dysfunction in Diabetes Mellitus and Cardiometabolic Disease
- PMID: 33125468
- PMCID: PMC7846151
- DOI: 10.1210/endrev/bnaa025
Microvascular Dysfunction in Diabetes Mellitus and Cardiometabolic Disease
Abstract
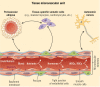
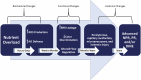
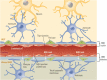
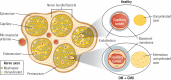
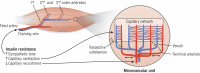
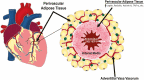
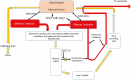
This review takes an inclusive approach to microvascular dysfunction in diabetes mellitus and cardiometabolic disease. In virtually every organ, dynamic interactions between the microvasculature and resident tissue elements normally modulate vascular and tissue function in a homeostatic fashion. This regulation is disordered by diabetes mellitus, by hypertension, by obesity, and by dyslipidemia individually (or combined in cardiometabolic disease), with dysfunction serving as an early marker of change. In particular, we suggest that the familiar retinal, renal, and neural complications of diabetes mellitus are late-stage manifestations of microvascular injury that begins years earlier and is often abetted by other cardiometabolic disease elements (eg, hypertension, obesity, dyslipidemia). We focus on evidence that microvascular dysfunction precedes anatomic microvascular disease in these organs as well as in heart, muscle, and brain. We suggest that early on, diabetes mellitus and/or cardiometabolic disease can each cause reversible microvascular injury with accompanying dysfunction, which in time may or may not become irreversible and anatomically identifiable disease (eg, vascular basement membrane thickening, capillary rarefaction, pericyte loss, etc.). Consequences can include the familiar vision loss, renal insufficiency, and neuropathy, but also heart failure, sarcopenia, cognitive impairment, and escalating metabolic dysfunction. Our understanding of normal microvascular function and early dysfunction is rapidly evolving, aided by innovative genetic and imaging tools. This is leading, in tissues like the retina, to testing novel preventive interventions at early, reversible stages of microvascular injury. Great hope lies in the possibility that some of these interventions may develop into effective therapies.
Keywords: cardiometabolic disease; diabetes complications; diabetes mellitus; microvascular rarefaction; microvessels; vascular endothelium.
© The Author(s) 2020. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

