Characterization of the tumor immune microenvironment in human papillomavirus-positive and -negative head and neck squamous cell carcinomas
- PMID: 33125511
- PMCID: PMC8188514
- DOI: 10.1007/s00262-020-02747-w
Characterization of the tumor immune microenvironment in human papillomavirus-positive and -negative head and neck squamous cell carcinomas
Abstract
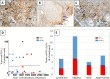
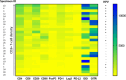
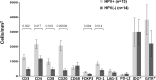
Approximately 15% of advanced head and neck squamous cell carcinomas (HNSCC) respond to anti-PD-(L)1 monotherapies. Tumor PD-L1 expression and human papillomavirus (HPV) status have been proposed as biomarkers to identify patients likely to benefit from these treatments. We aimed to understand the potential immune effects of HPV in HNSCC and to characterize additional potentially targetable immune-regulatory pathways in primary, treatment-naïve tumors. CD3, CD4, CD8, CD20, CD68, FoxP3, PD-1, PD-L2, LAG-3, IDO-1, and GITR cell densities were determined in 27 HNSCC specimens. IHC for PD-L1 assessed percentage of positive tumor cells and immune cells separately or as a combined positive score (CPS), and whether PD-L1 was expressed in an adaptive or constitutive pattern (i.e., PD-L1+ tumor cells juxtaposed to TILs or in the absence of TILs, respectively). HPV testing with p16 IHC was confirmed by HPV genotyping. When compared to HPV(-) tumors (n = 14), HPV+ tumors (n = 13) contained significantly higher densities of CD3+, CD4+, CD8+, CD20+, and PD-1+ cells (P < 0.02), and there was a trend towards increased density of FoxP3 + cells. PD-L1 expression patterns did not vary by tumor viral status, suggesting possible heterogeneous mechanisms driving constitutive vs adaptive PD-L1 expression patterns in HNSCC. IDO-1 expression was abundant (> 500 IDO-1+ cells/mm2 in 17/27 specimens) and was found on tumor cells as well as immune cells in 12/27 (44%) cases (range 5-80% tumor cells+). Notably, the studied markers varied on a per-patient basis and were not always related to the degree of T cell infiltration. These findings may inform therapeutic co-targeting strategies and raise consideration for a personalized treatment approach.
Keywords: GITR; HPV; Head and neck squamous cell carcinoma (HNSCC); IDO; PD-1; PD-L1.
Conflict of interest statement
J. M. Taube reports consulting/advisory board for BMS, Merck, AstraZeneca, and Compugen; research funding through Bristol Myers Squibb; and reagents and machine loan from Akoya Biosciences. S. L. Topalian reports stock and other ownership interests in Aduro Biotech, DNAtrix, Dracen Pharmaceuticals, Dragonfly Therapeutics, Ervaxx, Five Prime Therapeutics, Potenza Therapeutics, RAPT, Tizona Therapeutics, Trieza Therapeutics, and WindMIL; a consulting or advisory role in Amgen, DNAtrix, Dragonfly Therapeutics, Dynavax, Ervaxx, Five Prime Therapeutics, Immunocore, Immunomic Therapeutics, Janssen Pharmaceuticals, MedImmune/AstraZeneca, Merck, RAPT, and WindMIL; research grants from Bristol Myers Squibb and Compugen; patents, royalties, and/or other intellectual property through her institution with Aduro Biotech, Arbor Pharmaceuticals, Bristol Myers Squibb, Immunomic Therapeutics, NexImmune, and WindMIL; and travel, accommodations, and expenses from Bristol-Myers Squibb and Five Prime Therapeutics. P. Kvistborg is a consultant for Neon Therapeutics and Personalis and a recipient of grant/research support from Bristol-Myers Squibb and Merck. J. Haanen: NKI received financial compensation for advisory role of J. Haanen with AZ, Amgen, Bayer, BMS, Celsius Therapeutics, MSD, Merck Serono, Pfizer, Roche/Genentech, Neon Therapeutics, lmmunocore, Seattle Genetics, Novartis, GSK. Also, NKI received research grants through J. Haanen from BMS, MSD, Novartis, and Neon Therapeutics. J. Stein reports consulting (uncompensated) for AstraZeneca. No potential conflicts of interest were disclosed by the other authors.
Figures


Similar articles
-
PD-L1 expression correlates with tumor-infiltrating lymphocytes and better prognosis in patients with HPV-negative head and neck squamous cell carcinomas.Cancer Immunol Immunother. 2020 Oct;69(10):2089-2100. doi: 10.1007/s00262-020-02604-w. Epub 2020 May 24. Cancer Immunol Immunother. 2020. PMID: 32448984 Free PMC article.
-
HPV-positive status associated with inflamed immune microenvironment and improved response to anti-PD-1 therapy in head and neck squamous cell carcinoma.Sci Rep. 2019 Sep 16;9(1):13404. doi: 10.1038/s41598-019-49771-0. Sci Rep. 2019. PMID: 31527697 Free PMC article.
-
Programmed Death-1/Programmed Death-Ligand 1-Axis Blockade in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma Stratified by Human Papillomavirus Status: A Systematic Review and Meta-Analysis.Front Immunol. 2021 Apr 7;12:645170. doi: 10.3389/fimmu.2021.645170. eCollection 2021. Front Immunol. 2021. PMID: 33897693 Free PMC article.
-
Characterization of tumor-associated T-lymphocyte subsets and immune checkpoint molecules in head and neck squamous cell carcinoma.Oncotarget. 2017 Jul 4;8(27):44418-44433. doi: 10.18632/oncotarget.17901. Oncotarget. 2017. PMID: 28574843 Free PMC article.
-
The prognostic role of PD-L1 expression for survival in head and neck squamous cell carcinoma: A systematic review and meta-analysis.Oral Oncol. 2018 Nov;86:81-90. doi: 10.1016/j.oraloncology.2018.09.016. Epub 2018 Sep 17. Oral Oncol. 2018. PMID: 30409325
Cited by
-
Future investigative directions for novel therapeutic targets in head and neck cancer.Expert Rev Anticancer Ther. 2024 Nov;24(11):1067-1084. doi: 10.1080/14737140.2024.2417038. Epub 2024 Oct 16. Expert Rev Anticancer Ther. 2024. PMID: 39412140 Review.
-
How Risk Factors Affect Head and Neck Squamous Cell Carcinoma (HNSCC) Tumor Immune Microenvironment (TIME): Their Influence on Immune Escape Mechanisms and Immunotherapy Strategy.Biomedicines. 2022 Oct 7;10(10):2498. doi: 10.3390/biomedicines10102498. Biomedicines. 2022. PMID: 36289760 Free PMC article. Review.
-
Antiviral Responses in Cancer: Boosting Antitumor Immunity Through Activation of Interferon Pathway in the Tumor Microenvironment.Front Immunol. 2021 Dec 2;12:782852. doi: 10.3389/fimmu.2021.782852. eCollection 2021. Front Immunol. 2021. PMID: 34925363 Free PMC article. Review.
-
Identification and Validation of an Apoptosis-Related Gene Prognostic Signature for Oral Squamous Cell Carcinoma.Front Oncol. 2022 Jun 13;12:889049. doi: 10.3389/fonc.2022.889049. eCollection 2022. Front Oncol. 2022. PMID: 35769708 Free PMC article.
-
Why responses to immune checkpoint inhibitors are heterogeneous in head and neck cancers: Contributions from tumor-intrinsic and host-intrinsic factors.Front Oncol. 2022 Oct 18;12:995434. doi: 10.3389/fonc.2022.995434. eCollection 2022. Front Oncol. 2022. PMID: 36330485 Free PMC article. Review.
References
-
- Chow LQ, Haddad R, Gupta S, Mahipal A, Mehra R, Tahara M, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. 2016;34:3838–3845. doi: 10.1200/JCO.2016.68.1478. - DOI - PMC - PubMed
-
- Cohen EEW, Soulieres D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393:156–167. doi: 10.1016/S0140-6736(18)31999-8. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

