The use of patient-reported outcomes to detect adverse events in metastatic melanoma patients receiving immunotherapy: a randomized controlled pilot trial
- PMID: 33125537
- PMCID: PMC7599285
- DOI: 10.1186/s41687-020-00255-0
The use of patient-reported outcomes to detect adverse events in metastatic melanoma patients receiving immunotherapy: a randomized controlled pilot trial
Abstract
Background: A randomized controlled pilot trial was conducted to assess if melanoma patients treated with immunotherapy had the number of grade 3 or 4 adverse events during treatment reduced by 50% using a tailored electronic patient-reported outcomes tool in addition to standard toxicity monitoring compared to standard monitoring alone. Secondary endpoints were: if more AEs were reported in the intervention group, if there was a difference between the two groups in the number of telephone consultations, extra out-patient visits, number of days in the hospital, days in steroid treatment and the time patients experienced grade 2 or higher toxicity.
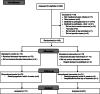
Patients and methods: Melanoma patients receiving immunotherapy at the Department of Oncology, Odense University Hospital, Denmark participated. Standard care included assessment of AEs by a clinician before each treatment cycle using the Common Terminology Criteria for Adverse Events. In addition, patients randomized to the intervention reported their AEs weekly by an electronic PRO-tool based on the PRO-CTCAE platform.
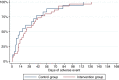
Results: One hundred forty-six melanoma patients were randomized. In this study, we did not detect a difference between the two groups in the number of grade 3 or 4 AEs (P = 0.983), in the overall number of AEs (P = 0.560) or in the time the patients in the two groups experienced grade 2 or higher toxicity (0.516). The number of phone contacts was significantly higher in the intervention group (P = 0.009) and there was a tendency towards patients in the intervention group having more extra visits (P = 0.156).
Conclusion: It has been examined if the number of severe AEs for melanoma patients receiving immunotherapy could be reduced by involving the patients in the reporting of symptoms. The results do not justify the expansion of the pilot study into a regular phase III study with this particular set-up. However, a significant difference in the number of phone contacts was found as patients in the intervention group called more frequently, indicating that their attention to AEs was increased. Even though the use of an electronic PRO tool could not reduce the number of severe AEs in this melanoma population, a positive impact on other endpoints such as QoL, communication, or treatment-planning, cannot be excluded.
Trial registration: Clinicaltrials.gov NCT03073031 Registered 8 March 2017, Retrospectively registered.
Keywords: Adverse events; E-health; Immunotherapy; Melanoma; PRO; Patient involvement; Patient-reported outcomes; RCT; Toxicity.
Conflict of interest statement
The authors indicated no potential conflict of interest.
Figures
References
-
- Matthews NH LW, Qureshi AA, et al. . Epidemiology of Melanoma. In: Ward WH, Farma JM, editors. Cutaneous Melanoma: Etiology and Therapy [Internet]. Brisbane (AU): Codon Publications; 2017 Dec 21. Chapter 1. Available from: https://www.ncbi.nlm.nih.gov/books/NBK481862/ 2017.
-
- KræftensBekæmpelse. Statistik om modermærkekræft https://www.cancer.dk/modermaerkekraeft-malignt-melanom/statistik-moderm.... Accessed 3 Dec 2019.
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical