Decarboxylative Alkyl Coupling Promoted by NADH and Blue Light
- PMID: 33125842
- PMCID: PMC7705967
- DOI: 10.1021/jacs.0c09678
Decarboxylative Alkyl Coupling Promoted by NADH and Blue Light
Abstract
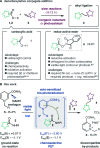
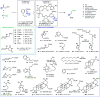
Photoexcited dihydronicotinamides like NADH and analogues have been found to generate alkyl radicals upon reductive decarboxylation of redox-active esters without auxiliary photocatalysts. This principle allowed aliphatic photocoupling between redox-active carboxylate derivatives and electron-poor olefins, displaying surprising water and air-tolerance and unusually high coupling rates in dilute conditions. The orthogonality of the reaction in the presence of other carboxylic acids and its utility in the functionalization of DNA is presented, notably using visible light in combination with NADH, the ubiquitous reductant of life.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Palladium-Catalyzed Decarboxylative Heck-Type Coupling of Activated Aliphatic Carboxylic Acids Enabled by Visible Light.Chemistry. 2018 Mar 26;24(18):4552-4555. doi: 10.1002/chem.201800813. Epub 2018 Mar 9. Chemistry. 2018. PMID: 29451724
-
Visible-Light-Mediated Dual Decarboxylative Coupling of Redox-Active Esters with α,β-Unsaturated Carboxylic Acids.Chemistry. 2017 Aug 1;23(43):10259-10263. doi: 10.1002/chem.201702200. Epub 2017 Jul 13. Chemistry. 2017. PMID: 28631846
-
Visible Light-Induced Decarboxylative Alkylation of Heterocyclic Aromatics with Carboxylic Acids via Anthocyanin as a Photocatalyst.Chem Asian J. 2020 Jul 1;15(13):1976-1981. doi: 10.1002/asia.202000277. Epub 2020 May 27. Chem Asian J. 2020. PMID: 32385937
-
Visible-Light-Photosensitized Aryl and Alkyl Decarboxylative Functionalization Reactions.Angew Chem Int Ed Engl. 2019 Jul 29;58(31):10514-10520. doi: 10.1002/anie.201904671. Epub 2019 Jul 3. Angew Chem Int Ed Engl. 2019. PMID: 31162874 Review.
-
Cost-Effective Carbon Quaternization with Redox-Active Esters and Olefins.Angew Chem Int Ed Engl. 2024 Oct 7;63(41):e202408301. doi: 10.1002/anie.202408301. Epub 2024 Sep 2. Angew Chem Int Ed Engl. 2024. PMID: 38982711 Review.
Cited by
-
Developments in Photoredox-Mediated Alkylation for DNA-Encoded Libraries.Trends Chem. 2021 Mar;3(3):161-175. doi: 10.1016/j.trechm.2020.11.010. Epub 2020 Dec 29. Trends Chem. 2021. PMID: 33987530 Free PMC article.
-
On-DNA Hydroalkylation to Introduce Diverse Bicyclo[1.1.1]pentanes and Abundant Alkyls via Halogen Atom Transfer.J Am Chem Soc. 2022 Jul 13;144(27):12184-12191. doi: 10.1021/jacs.2c03025. Epub 2022 Jun 27. J Am Chem Soc. 2022. PMID: 35759692 Free PMC article.
-
Photoredox-mediated hydroalkylation and hydroarylation of functionalized olefins for DNA-encoded library synthesis.Chem Sci. 2021 Aug 5;12(36):12036-12045. doi: 10.1039/d1sc03191k. eCollection 2021 Sep 22. Chem Sci. 2021. PMID: 34667569 Free PMC article.
-
Photochemical Methods Applied to DNA Encoded Library (DEL) Synthesis.Acc Chem Res. 2023 Feb 7;56(3):385-401. doi: 10.1021/acs.accounts.2c00778. Epub 2023 Jan 19. Acc Chem Res. 2023. PMID: 36656960 Free PMC article.
-
Facile enantioselective synthesis of multi-substituted norbornanes/norbornenes using a latent synthon strategy.Nat Commun. 2024 Sep 27;15(1):8351. doi: 10.1038/s41467-024-52644-4. Nat Commun. 2024. PMID: 39333126 Free PMC article.
References
-
- Ankenbruck N.; Courtney T.; Naro Y.; Deiters A. Optochemical Control of Biological Processes in Cells and Animals. Angew. Chem., Int. Ed. 2018, 57 (11), 2768–2798. 10.1002/anie.201700171. - DOI - PMC - PubMed
- Fehrentz T.; Schönberger M.; Trauner D. Optochemical Genetics. Angew. Chem., Int. Ed. 2011, 50 (51), 12156–12182. 10.1002/anie.201103236. - DOI - PubMed
- Albert L.; Vázquez O. Photoswitchable Peptides for Spatiotemporal Control of Biological Functions. Chem. Commun. 2019, 55 (69), 10192–10213. 10.1039/C9CC03346G. - DOI - PubMed
- Szymański W.; Beierle J. M.; Kistemaker H. A. V.; Velema W. A.; Feringa B. L. Reversible Photocontrol of Biological Systems by the Incorporation of Molecular Photoswitches. Chem. Rev. 2013, 113 (8), 6114–6178. 10.1021/cr300179f. - DOI - PubMed
- Heinrich B.; Bouazoune K.; Wojcik M.; Bakowsky U.; Vázquez O. ortho-Fluoroazobenzene Derivatives as DNA Intercalators for Photocontrol of DNA and Nucleosome Binding by Visible Light. Org. Biomol. Chem. 2019, 17 (7), 1827–1833. 10.1039/C8OB02343C. - DOI - PubMed
- Broichhagen J.; Trauner D. The in vivo Chemistry of Photoswitched Tethered Ligands. Curr. Opin. Chem. Biol. 2014, 21, 121–127. 10.1016/j.cbpa.2014.07.008. - DOI - PubMed
-
- Klán P.; Šolomek T.; Bochet C. G.; Blanc A.; Givens R.; Rubina M.; Popik V.; Kostikov A.; Wirz J. Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy. Chem. Rev. 2013, 113 (1), 119–191. 10.1021/cr300177k. - DOI - PMC - PubMed
- Kolarski D.; Sugiyama A.; Breton G.; Rakers C.; Ono D.; Schulte A.; Tama F.; Itami K.; Szymanski W.; Hirota T.; Feringa B. L. Controlling the Circadian Clock with High Temporal Resolution through Photodosing. J. Am. Chem. Soc. 2019, 141 (40), 15784–15791. 10.1021/jacs.9b05445. - DOI - PMC - PubMed
-
-
For examples of nonlight regulated bioorthogonal reactions, see:
- Sletten E. M.; Bertozzi C. R. Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality. Angew. Chem., Int. Ed. 2009, 48 (38), 6974–6998. 10.1002/anie.200900942. - DOI - PMC - PubMed
- Patterson D. M.; Nazarova L. A.; Prescher J. A. Finding the Right (Bioorthogonal) Chemistry. ACS Chem. Biol. 2014, 9 (3), 592–605. 10.1021/cb400828a. - DOI - PubMed
- Whiting M.; Muldoon J.; Lin Y.-C.; Silverman S. M.; Lindstrom W.; Olson A. J.; Kolb H. C.; Finn M. G.; Sharpless K. B.; Elder J. H.; Fokin V. V. Inhibitors of HIV-1 Protease by Using In Situ Click Chemistry. Angew. Chem., Int. Ed. 2006, 45 (9), 1435–1439. 10.1002/anie.200502161. - DOI - PubMed
- Fang Y.; Zhang H.; Huang Z.; Scinto S. L.; Yang J. C.; Am Ende C. W.; Dmitrenko O.; Johnson D. S.; Fox J. M. Photochemical Syntheses, Transformations, and Bioorthogonal Chemistry of Trans-Cycloheptene and Sila Trans-Cycloheptene Ag(I) complexes. Chem. Sci. 2018, 9 (7), 1953–1963. 10.1039/C7SC04773H. - DOI - PMC - PubMed
- An P.; Lewandowski T. M.; Erbay T. G.; Liu P.; Lin Q. Sterically Shielded, Stabilized Nitrile Imine for Rapid Bioorthogonal Protein Labeling in Live Cells. J. Am. Chem. Soc. 2018, 140 (14), 4860–4868. 10.1021/jacs.8b00126. - DOI - PMC - PubMed
- Blackman M. L.; Royzen M.; Fox J. M. Tetrazine Ligation: Fast Bioconjugation Based on Inverse-Electron-Demand Diels–Alder Reactivity. J. Am. Chem. Soc. 2008, 130 (41), 13518–13519. 10.1021/ja8053805. - DOI - PMC - PubMed
- Patterson D. M.; Nazarova L. A.; Xie B.; Kamber D. N.; Prescher J. A. Functionalized Cyclopropenes As Bioorthogonal Chemical Reporters. J. Am. Chem. Soc. 2012, 134 (45), 18638–18643. 10.1021/ja3060436. - DOI - PubMed
- Sachdeva A.; Wang K.; Elliott T.; Chin J. W. Concerted, Rapid, Quantitative, and Site-Specific Dual Labeling of Proteins. J. Am. Chem. Soc. 2014, 136 (22), 7785–7788. 10.1021/ja4129789. - DOI - PMC - PubMed
- Laughlin S. T.; Baskin J. M.; Amacher S. L.; Bertozzi C. R. In Vivo Imaging of Membrane-Associated Glycans in Developing Zebrafish. Science 2008, 320 (5876), 664–667. 10.1126/science.1155106. - DOI - PMC - PubMed
-
-
-
For examples of light-regulated bioorthogonal reactions:
- Tasdelen M. A.; Yagci Y. Light-Induced Click Reactions. Angew. Chem., Int. Ed. 2013, 52 (23), 5930–5938. 10.1002/anie.201208741. - DOI - PubMed
- Chen R. T.; Marchesan S.; Evans R. A.; Styan K. E.; Such G. K.; Postma A.; McLean K. M.; Muir B. W.; Caruso F. Photoinitiated Alkyne–Azide Click and Radical Cross-Linking Reactions for the Patterning of PEG Hydrogels. Biomacromolecules 2012, 13 (3), 889–895. 10.1021/bm201802w. - DOI - PubMed
- Singh K.; Fennell C. J.; Coutsias E. A.; Latifi R.; Hartson S.; Weaver J. D. Light Harvesting for Rapid and Selective Reactions: Click Chemistry with Strain-Loadable Alkenes. Chem. 2018, 4 (1), 124–137. 10.1016/j.chempr.2017.11.007. - DOI
- Kaur G.; Singh G.; Singh J. Photochemical Tuning of Materials: A Click Chemistry Perspective. Mater. Today Chem. 2018, 8, 56–84. 10.1016/j.mtchem.2018.03.002. - DOI
- Bordoni A. V.; Lombardo M. V.; Wolosiuk A. Photochemical radical thiol–ene click-based methodologies for silica and transition metal oxides materials chemical modification: a mini-review. RSC Adv. 2016, 6 (81), 77410–77426. 10.1039/C6RA10388J. - DOI
- Ramil C. P.; Lin Q. Photoclick chemistry: a fluorogenic light-triggered in vivo ligation reaction. Curr. Opin. Chem. Biol. 2014, 21, 89–95. 10.1016/j.cbpa.2014.05.024. - DOI - PMC - PubMed
-
Publication types
LinkOut - more resources
Full Text Sources

