Molecular Basis of Chemotactile Sensation in Octopus
- PMID: 33125889
- PMCID: PMC7605239
- DOI: 10.1016/j.cell.2020.09.008
Molecular Basis of Chemotactile Sensation in Octopus
Abstract
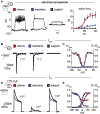
Animals display wide-ranging evolutionary adaptations based on their ecological niche. Octopuses explore the seafloor with their flexible arms using a specialized "taste by touch" system to locally sense and respond to prey-derived chemicals and movement. How the peripherally distributed octopus nervous system mediates relatively autonomous arm behavior is unknown. Here, we report that octopus arms use a family of cephalopod-specific chemotactile receptors (CRs) to detect poorly soluble natural products, thereby defining a form of contact-dependent, aquatic chemosensation. CRs form discrete ion channel complexes that mediate the detection of diverse stimuli and transduction of specific ionic signals. Furthermore, distinct chemo- and mechanosensory cells exhibit specific receptor expression and electrical activities to support peripheral information coding and complex chemotactile behaviors. These findings demonstrate that the peripherally distributed octopus nervous system is a key site for signal processing and highlight how molecular and anatomical features synergistically evolve to suit an animal's environmental context.
Keywords: chemosensation; evolution; ion channels; neuroethology; neuroscience; octopus; sensory physiology; signal transduction.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures





Comment in
-
A Sucker for Taste.Cell. 2020 Oct 29;183(3):587-588. doi: 10.1016/j.cell.2020.10.012. Cell. 2020. PMID: 33125886
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

