What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2
- PMID: 33125914
- PMCID: PMC7837315
- DOI: 10.1016/S1473-3099(20)30773-8
What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2
Abstract
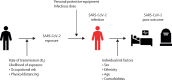
The novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has caused more than 1 million deaths in the first 6 months of the pandemic and huge economic and social upheaval internationally. An efficacious vaccine is essential to prevent further morbidity and mortality. Although some countries might deploy COVID-19 vaccines on the strength of safety and immunogenicity data alone, the goal of vaccine development is to gain direct evidence of vaccine efficacy in protecting humans against SARS-CoV-2 infection and COVID-19 so that manufacture of efficacious vaccines can be selectively upscaled. A candidate vaccine against SARS-CoV-2 might act against infection, disease, or transmission, and a vaccine capable of reducing any of these elements could contribute to disease control. However, the most important efficacy endpoint, protection against severe disease and death, is difficult to assess in phase 3 clinical trials. In this Review, we explore the challenges in assessing the efficacy of candidate SARS-CoV-2 vaccines, discuss the caveats needed to interpret reported efficacy endpoints, and provide insight into answering the seemingly simple question, "Does this COVID-19 vaccine work?"
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures

References
-
- World Bank . World Bank; Washington, DC: 2020. Global economic prospects.
-
- Gavi The Gavi COVAX AMC: an investment opportunity. https://www.gavi.org/covax-facility
-
- WHO . World Health Organization; Geneva: 2020. Draft landscape of COVID-19 candidate vaccines.
-
- WHO . World Health Organisation; Geneva: 2020. 2019 novel coronavirus (2019-nCoV): strategic preparedness and response plan.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

