Resolving the paradox of ferroptotic cell death: Ferrostatin-1 binds to 15LOX/PEBP1 complex, suppresses generation of peroxidized ETE-PE, and protects against ferroptosis
- PMID: 33126055
- PMCID: PMC7596334
- DOI: 10.1016/j.redox.2020.101744
Resolving the paradox of ferroptotic cell death: Ferrostatin-1 binds to 15LOX/PEBP1 complex, suppresses generation of peroxidized ETE-PE, and protects against ferroptosis
Abstract
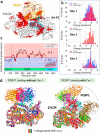
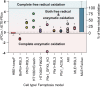
Hydroperoxy-eicosatetraenoyl-phosphatidylethanolamine (HpETE-PE) is a ferroptotic cell death signal. HpETE-PE is produced by the 15-Lipoxygenase (15LOX)/Phosphatidylethanolamine Binding Protein-1 (PEBP1) complex or via an Fe-catalyzed non-enzymatic radical reaction. Ferrostatin-1 (Fer-1), a common ferroptosis inhibitor, is a lipophilic radical scavenger but a poor 15LOX inhibitor arguing against 15LOX having a role in ferroptosis. In the current work, we demonstrate that Fer-1 does not affect 15LOX alone, however, it effectively inhibits HpETE-PE production by the 15LOX/PEBP1 complex. Computational molecular modeling shows that Fer-1 binds to the 15LOX/PEBP1 complex at three sites and could disrupt the catalytically required allosteric motions of the 15LOX/PEBP1 complex. Using nine ferroptosis cell/tissue models, we show that HpETE-PE is produced by the 15LOX/PEBP1 complex and resolve the long-existing Fer-1 anti-ferroptotic paradox.
Keywords: 15-Lipoxygenase; Ferroptosis; Ferrostatin-1; Hydroperoxy-eicosatetraenoyl-phosphatidylethanolamines; Phospholipid peroxidation.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Figures




References
-
- Bayır H., Anthonymuthu T.S., Tyurina Y.Y., Patel S.J., Amoscato A.A., Lamade A.M., Yang Q., Vladimirov G.K., Philpott C.C., Kagan V.E. Achieving life through death: redox biology of lipid peroxidation in ferroptosis. Cell Chem. Biol. 2020;27:387–408. doi: 10.1016/j.chembiol.2020.03.014. - DOI - PMC - PubMed
-
- Cozza G., Rossetto M., Bosello-Travain V., Maiorino M., Roveri A., Toppo S., Zaccarin M., Zennaro L., Ursini F. Glutathione peroxidase 4-catalyzed reduction of lipid hydroperoxides in membranes: the polar head of membrane phospholipids binds the enzyme and addresses the fatty acid hydroperoxide group toward the redox center. Free Radic. Biol. Med. 2017;112:1–11. doi: 10.1016/j.freeradbiomed.2017.07.010. - DOI - PubMed
-
- Kagan V.E., Mao G., Qu F., Angeli J.P., Doll S., Croix C.S., Dar H.H., Liu B., Tyurin V.A., Ritov V.B., Kapralov A.A., Amoscato A.A., Jiang J., Anthonymuthu T., Mohammadyani D., Yang Q., Proneth B., Klein-Seetharaman J., Watkins S., Bahar I., Greenberger J., Mallampalli R.K., Stockwell B.R., Tyurina Y.Y., Conrad M., Bayir H. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

