Anti-SARS-CoV-2 IgG antibody response among Indian COVID-19 patients using β-propiolactone-inactivated, whole virus-based indirect ELISA
- PMID: 33126149
- PMCID: PMC7581401
- DOI: 10.1016/j.jviromet.2020.113996
Anti-SARS-CoV-2 IgG antibody response among Indian COVID-19 patients using β-propiolactone-inactivated, whole virus-based indirect ELISA
Abstract
Background: Coronavirus disease 2019 (COVID-19) pandemic caused by infection with severe acute respiratory syndrome - coronavirus-2 (SARS-CoV-2) continues to affect many countries and large populations. Serologic assays for antibody detection aid patient diagnosis and seroepidemiologic investigations.
Methods: An indirect IgG ELISA was developed indigenously using β-propiolactone (BPL) inactivated SARS-CoV-2. This assay was used for screening 200 healthy donor sera collected prior to COVID-19 emergence (2017-2019), 185 serum/plasma samples of confirmed COVID-19 patients (n = 137) and 57 samples of viral RNA positive asymptomatic contacts (n = 51). The IgG response was studied in relation to duration and severity of illness.
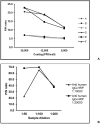
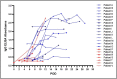
Results: The ELISA demonstrated 97 % specificity and IgG detection in >50 %, 80 %, 93.8 % and 100 % of the patients respectively during the first, second, third and fourth week of illness. IgG detection rate was higher in patients with severe disease (SD, 90.9 %) than those with mild disease (MD, 68.8 %) during the second week of illness (P = 0.027). IgG seropositivity among asymptomatic contacts was 64.7 %. IgG ELISA absorbance values were higher in SD than MD patients during the first 2 weeks of illness (P < 0.05). No significant difference was observed between the absorbance values of asymptomatic subjects and MD patients (P = 0.94).
Conclusion: The BPL inactivated virus-based ELISA could detect IgG antibodies early and in a significant proportion of COVID-19 patients suggesting its potential utility as a supplement to the currently used viral RNA detection tests in patient diagnosis and contact screening algorithms.
Keywords: Antibody; COVID-19; ELISA; IgG; Inactivated virus; SARS-CoV-2.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors report no declarations of interest.
Figures


References
-
- Guo L., Ren L., Yang S., Xiao M., Chang D., Yang F., Dela Cruz C.S., Wang Y., Wu C., Xiao Y., Zhang L., Han L., Dang S., Xu Y., Yang Q., Xu S., Zhu H., Xu Y., Jin Q., Sharma L., Wang L., Wang J. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) Clin. Infect. Dis. 2020;(March):21. doi: 10.1093/cid/ciaa310. ciaa310. - DOI - PMC - PubMed
-
- Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., Wang X., Yuan J., Li T., Li J., Qian S., Hong C., Wang F., Liu Y., Wang Z., He Q., Li Z., He B., Zhang T., Fu Y., Ge S., Liu L., Zhang J., Xia N., Zhang Z. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin. Infect. Dis. 2020;(March):28. doi: 10.1093/cid/ciaa344. ciaa344. - DOI - PMC - PubMed
-
- Liu W., Liu L., Kou G., Zheng Y., Ding Y., Ni W., Wang Q., Tan L., Wu W., Tang S., Xiong Z., Zheng S. Evaluation of Nucleocapsid and Spike protein-based enzyme-linked immunosorbent assays for detecting antibodies against SARS-CoV-2. J. Clin. Microbiol. 2020;58(May (6)) doi: 10.1128/JCM.00461-20. e00461-20. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

