The Role of Complement in the Mechanism of Action of Therapeutic Anti-Cancer mAbs
- PMID: 33126570
- PMCID: PMC7709112
- DOI: 10.3390/antib9040058
The Role of Complement in the Mechanism of Action of Therapeutic Anti-Cancer mAbs
Abstract
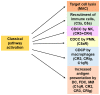
Unconjugated anti-cancer IgG1 monoclonal antibodies (mAbs) activate antibody-dependent cellular cytotoxicity (ADCC) by natural killer (NK) cells and antibody-dependent cellular phagocytosis (ADCP) by macrophages, and these activities are thought to be important mechanisms of action for many of these mAbs in vivo. Several mAbs also activate the classical complement pathway and promote complement-dependent cytotoxicity (CDC), although with very different levels of efficacy, depending on the mAb, the target antigen, and the tumor type. Recent studies have unraveled the various structural factors that define why some IgG1 mAbs are strong mediators of CDC, whereas others are not. The role of complement activation and membrane inhibitors expressed by tumor cells, most notably CD55 and CD59, has also been quite extensively studied, but how much these affect the resistance of tumors in vivo to IgG1 therapeutic mAbs still remains incompletely understood. Recent studies have demonstrated that complement activation has multiple effects beyond target cell lysis, affecting both innate and adaptive immunity mediated by soluble complement fragments, such as C3a and C5a, and by stimulating complement receptors expressed by immune cells, including NK cells, neutrophils, macrophages, T cells, and dendritic cells. Complement activation can enhance ADCC and ADCP and may contribute to the vaccine effect of mAbs. These different aspects of complement are also briefly reviewed in the specific context of FDA-approved therapeutic anti-cancer IgG1 mAbs.
Keywords: antibody dependent cellular cytotoxicity; complement; complement receptors; phagocytosis; therapeutic monoclonal antibodies (mAbs).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Lindorfer M.A., Koehl J., Taylor R.P. Interactions between the complement system and Fcgamma receptors. In: Nimmerhahn F., Ackerman M.E., editors. IgG Fc: Linking Adaptive and Innate Immunity. Elsevier Press; Amsterdam, The Netherlands: 2013. pp. 49–74.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

