A Universal Pharmacokinetic Model for Dexmedetomidine in Children and Adults
- PMID: 33126702
- PMCID: PMC7692360
- DOI: 10.3390/jcm9113480
A Universal Pharmacokinetic Model for Dexmedetomidine in Children and Adults
Abstract
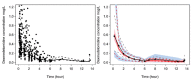
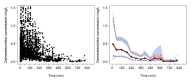
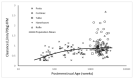
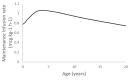
A universal pharmacokinetic model was developed from pooled paediatric and adult data (40.6 postmenstrual weeks, 70.8 years, 3.1-152 kg). A three-compartment pharmacokinetic model with first-order elimination was superior to a two-compartment model to describe these pooled dexmedetomidine data. Population parameter estimates (population parameter variability%) were clearance (CL) 0.9 L/min/70 kg (36); intercompartmental clearances (Q2) 1.68 L/min/70 kg (63); Q3 0.62 L/min/70 kg (90); volume of distribution in the central compartment (V1) 25.2 L/70 kg (103.9); rapidly equilibrating peripheral compartment (V2) 34.4 L/70 kg (41.8); slow equilibrating peripheral compartment (V3) 65.4 L/70 kg (62). Obesity was best described by fat-free mass for clearances and normal fat mass for volumes with a factor for fat mass (FfatV) of 0.293. Models describing dexmedetomidine pharmacokinetics in adults can be applied to children by accounting for size (allometry) and age (maturation). This universal dexmedetomidine model is applicable to a broad range of ages and weights: neonates through to obese adults. Lean body weight is a better size descriptor for dexmedetomidine clearance than total body weight. This parameter set could be programmed into target-controlled infusion pumps for use in a broad population.
Keywords: anaesthesia; children; dexmedetomidine; intravenous; obesity; pharmacodynamics; pharmacokinetics; target-controlled infusion; total intravenous anaesthesia (TIVA).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Minto C.F., Schnider T.W., Egan T.D., Youngs E., Lemmens H.J., Gambus P.L., Billard V., Hoke J.F., Moore K.H., Hermann D.J., et al. Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil. I. Model development. Anesthesiology. 1997;86:10–23. doi: 10.1097/00000542-199701000-00004. - DOI - PubMed
LinkOut - more resources
Full Text Sources

