Modelling Pancreatic Neuroendocrine Cancer: From Bench Side to Clinic
- PMID: 33126717
- PMCID: PMC7693644
- DOI: 10.3390/cancers12113170
Modelling Pancreatic Neuroendocrine Cancer: From Bench Side to Clinic
Abstract
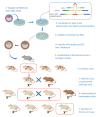
Pancreatic neuroendocrine tumours (pNETs) are a heterogeneous group of epithelial tumours with neuroendocrine differentiation. Although rare (incidence of <1 in 100,000), they are the second most common group of pancreatic neoplasms after pancreatic ductal adenocarcinoma (PDAC). pNET incidence is however on the rise and patient outcomes, although variable, have been linked with 5-year survival rates as low as 40%. Improvement of diagnostic and treatment modalities strongly relies on disease models that reconstruct the disease ex vivo. A key constraint in pNET research, however, is the absence of human pNET models that accurately capture the original tumour phenotype. In attempts to more closely mimic the disease in its native environment, three-dimensional culture models as well as in vivo models, such as genetically engineered mouse models (GEMMs), have been developed. Despite adding significant contributions to our understanding of more complex biological processes associated with the development and progression of pNETs, factors such as ethical considerations and low rates of clinical translatability limit their use. Furthermore, a role for the site-specific extracellular matrix (ECM) in disease development and progression has become clear. Advances in tissue engineering have enabled the use of tissue constructs that are designed to establish disease ex vivo within a close to native ECM that can recapitulate tumour-associated tissue remodelling. Yet, such advanced models for studying pNETs remain underdeveloped. This review summarises the most clinically relevant disease models of pNETs currently used, as well as future directions for improved modelling of the disease.
Keywords: disease models; genetically engineered mouse models; multicellular spheroids; organoids; pancreatic cancer; pancreatic neuroendocrine tumours.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Falconi M., Eriksson B., Kaltsas G., Bartsch D., Capdevila J.A., Caplin M., Kos-Kudla B., Kwekkeboom D.J., Rindi G., Kloppel G., et al. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology. 2016;103:153–171. doi: 10.1159/000443171. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

