Altered Antibody Responses in Persons Infected with HIV-1 While Using Preexposure Prophylaxis
- PMID: 33126825
- PMCID: PMC8020499
- DOI: 10.1089/AID.2020.0137
Altered Antibody Responses in Persons Infected with HIV-1 While Using Preexposure Prophylaxis
Abstract
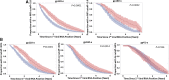
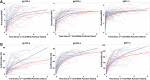
Preexposure prophylaxis (PrEP) is an effective HIV prevention tool, although effectiveness is dependent upon adherence. It is important to characterize the impact of PrEP on HIV antibody responses in people who experience breakthrough infections to understand the potential impact on timely diagnosis and treatment. Longitudinal HIV-1-specific antibody responses were evaluated in 42 people who inject drugs (PWID) from the Bangkok Tenofovir Study (BTS) (placebo = 28; PrEP = 14) who acquired HIV while receiving PrEP. HIV-1 antibody levels and avidity to three envelope proteins (gp41, gp160, and gp120) were measured in the plasma using a customized Bio-Plex (Bio-Rad Laboratories, Hercules, CA) assay. A time-to-event analysis was performed for each biomarker to compare the distribution of times at which study subjects exceeded the recent/long-term assay threshold, comparing PrEP and placebo treatment groups. We fit mixed-effects models to identify longitudinal differences in antibody levels and avidity between groups. Overall, longitudinal antibody levels and avidity were notably lower in the PrEP breakthrough group compared to the placebo group. Time-to-event analyses demonstrated a difference in time to antibody reactivity between treatment groups for all Bio-Plex biomarkers. Longitudinal gp120 antibody levels within the PrEP breakthrough group were decreased compared to the placebo group. When accounting for PrEP adherence, both gp120 and gp160 antibody levels were lower in the PrEP breakthrough group compared to the placebo group. We demonstrate hindered envelope antibody maturation in PWID who became infected while receiving PrEP in the BTS, which has significant implications for HIV diagnosis. Delayed maturation of the antibody response to HIV may increase the time to detection for antibody-based tests. Clinical Trial Registration Number, NCT00119106.
Keywords: HIV; antibody maturation; preexposure prophylaxis; seroconversion.
Conflict of interest statement
J.G.G-L is named in U.S. Government patents on “Inhibition of HIV infection through chemoprophylaxis”, and in U.S. Government patent applications on “HIV postexposure prophylaxis” and “HIV pre-exposure prophylaxis”. The other authors declare no conflicts of interest.
Figures
References
-
- Thigpen MC, Kebaabetswe PM, Paxton LA, et al. : Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012;367:423–434 - PubMed
-
- Choopanya K, Martin M, Suntharasamai P, et al. : Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013;381:2083–2090 - PubMed
-
- World Health Organization: Guideline on When to Start Antiretroviral Therapy and on Pre-exposure Prophylaxis for HIV. Geneva, Switzerland: WHO, 2015 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

