Robust performance of a novel stool DNA test of methylated SDC2 for colorectal cancer detection: a multicenter clinical study
- PMID: 33126908
- PMCID: PMC7602331
- DOI: 10.1186/s13148-020-00954-x
Robust performance of a novel stool DNA test of methylated SDC2 for colorectal cancer detection: a multicenter clinical study
Abstract
Background and aims: Stool DNA testing is an emerging and attractive option for colorectal cancer (CRC) screening. We previously evaluated the feasibility of a stool DNA (sDNA) test of methylated SDC2 for CRC detection. The aim of this study was to assess its performance in a multicenter clinical trial setting.
Methods: Each participant was required to undergo a sDNA test and a reference colonoscopy. The sDNA test consists of quantitative assessment of methylation status of SDC2 promoter. Results of real-time quantitative methylation-specific PCR were dichotomized as positive and negative, and the main evaluation indexes were sensitivity, specificity, and kappa value. All sDNA tests were performed and analyzed independently of colonoscopy.
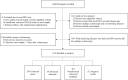
Results: Among the 1110 participants from three clinical sites analyzed, 359 and 38 were diagnosed, respectively, with CRC and advanced adenomas by colonoscopy. The sensitivity of the sDNA test was 301/359 (83.8%) for CRC, 16/38 (42.1%) for advanced adenomas, and 134/154 (87.0%) for early stage CRC (stage I-II). Detection rate did not vary significantly according to age, tumor location, differentiation, and TNM stage, except for gender. The follow-up testing of 40 postoperative patients with CRC returned negative results as their tumors had been surgically removed. The specificity of the sDNA test was 699/713 (98.0%), and unrelated cancers and diseases did not seem to interfere with the testing. The kappa value was 0.84, implying an excellent diagnostic consistency between the sDNA test and colonoscopy.
Conclusion: Noninvasive sDNA test using methylated SDC2 as the exclusive biomarker is a clinically viable and accurate CRC detection method.
Chinese clinical trial registry: Chi-CTR-TRC-1900026409, retrospectively registered on October 8, 2019; http://www.chictr.org.cn/edit.aspx?pid=43888&htm=4 .
Keywords: Advanced adenoma; Colorectal cancer; Methylation; Stool DNA test.
Conflict of interest statement
Xianglin Liu, Yan Qi, Feng Niu, Chunhua Chen, Xiaolin Wu, Xianshu Wang, and Hongzhi Zou are current employees from Creative Biosciences (Guangzhou) CO., Ltd. No potential conflicts of interests were disclosed by the other authors.
Figures


Similar articles
-
Early detection of colorectal cancer based on presence of methylated syndecan-2 (SDC2) in stool DNA.Clin Epigenetics. 2019 Mar 15;11(1):51. doi: 10.1186/s13148-019-0642-0. Clin Epigenetics. 2019. PMID: 30876480 Free PMC article. Clinical Trial.
-
A novel method for early detection of colorectal cancer based on detection of methylation of two fragments of syndecan-2 (SDC2) in stool DNA.BMC Gastroenterol. 2022 Apr 18;22(1):191. doi: 10.1186/s12876-022-02264-3. BMC Gastroenterol. 2022. PMID: 35436855 Free PMC article.
-
A Stool DNA-Based SDC2 Methylation Test for the Early Detection of Colorectal Cancer in an Asymptomatic, High-Risk Population: A Multicenter Prospective Randomized Trial.Am J Gastroenterol. 2025 Mar 1;120(3):614-622. doi: 10.14309/ajg.0000000000003044. Epub 2024 Aug 21. Am J Gastroenterol. 2025. PMID: 39292188 Clinical Trial.
-
PPV and Detection Rate of mt-sDNA Testing, FIT, and CT Colonography for Advanced Neoplasia: A Hierarchic Bayesian Meta-Analysis of the Noninvasive Colorectal Screening Tests.AJR Am J Roentgenol. 2021 Oct;217(4):817-830. doi: 10.2214/AJR.20.25416. Epub 2021 Mar 11. AJR Am J Roentgenol. 2021. PMID: 33703913
-
Diagnostic value of stool DNA testing for multiple markers of colorectal cancer and advanced adenoma: a meta-analysis.Can J Gastroenterol. 2013 Aug;27(8):467-75. doi: 10.1155/2013/258030. Can J Gastroenterol. 2013. PMID: 23936877 Free PMC article. Review.
Cited by
-
Identification of Six Genes as Diagnostic Markers for Colorectal Cancer Detection by Integrating Multiple Expression Profiles.J Oncol. 2022 Jul 22;2022:3850674. doi: 10.1155/2022/3850674. eCollection 2022. J Oncol. 2022. PMID: 35909904 Free PMC article.
-
Genome-wide methylation profiling identify hypermethylated HOXL subclass genes as potential markers for esophageal squamous cell carcinoma detection.BMC Med Genomics. 2022 Nov 29;15(1):247. doi: 10.1186/s12920-022-01401-x. BMC Med Genomics. 2022. PMID: 36447287 Free PMC article.
-
A Comparison of Single and Combined Schemes of Asia-Pacific Colorectal Screening, Faecal Immunochemical and Stool Deoxyribonucleic Acid Testing for Community Colorectal Cancer Screening.J Multidiscip Healthc. 2023 Mar 1;16:571-586. doi: 10.2147/JMDH.S398997. eCollection 2023. J Multidiscip Healthc. 2023. PMID: 36883167 Free PMC article.
-
Value of faecal exfoliated cells in colorectal tumour screening using SDC2 methylation test.Ann Med. 2023;55(2):2261111. doi: 10.1080/07853890.2023.2261111. Epub 2023 Oct 2. Ann Med. 2023. PMID: 37783044 Free PMC article.
-
Current Research on Molecular Biomarkers for Colorectal Cancer in Stool Samples.Biology (Basel). 2023 Dec 27;13(1):15. doi: 10.3390/biology13010015. Biology (Basel). 2023. PMID: 38248446 Free PMC article. Review.
References
-
- International Agency for Research on Cancer. China source: Globocan 2018[R/OL]. [2019‐8‐1]. https://gco.iarc.fr/today/data/factsheets/populations/160‐china‐fact‐she....
-
- Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–573. doi: 10.1002/cncr.24760. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

