Robust performance of a novel stool DNA test of methylated SDC2 for colorectal cancer detection: a multicenter clinical study
- PMID: 33126908
- PMCID: PMC7602331
- DOI: 10.1186/s13148-020-00954-x
Robust performance of a novel stool DNA test of methylated SDC2 for colorectal cancer detection: a multicenter clinical study
Abstract
Background and aims: Stool DNA testing is an emerging and attractive option for colorectal cancer (CRC) screening. We previously evaluated the feasibility of a stool DNA (sDNA) test of methylated SDC2 for CRC detection. The aim of this study was to assess its performance in a multicenter clinical trial setting.
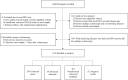
Methods: Each participant was required to undergo a sDNA test and a reference colonoscopy. The sDNA test consists of quantitative assessment of methylation status of SDC2 promoter. Results of real-time quantitative methylation-specific PCR were dichotomized as positive and negative, and the main evaluation indexes were sensitivity, specificity, and kappa value. All sDNA tests were performed and analyzed independently of colonoscopy.
Results: Among the 1110 participants from three clinical sites analyzed, 359 and 38 were diagnosed, respectively, with CRC and advanced adenomas by colonoscopy. The sensitivity of the sDNA test was 301/359 (83.8%) for CRC, 16/38 (42.1%) for advanced adenomas, and 134/154 (87.0%) for early stage CRC (stage I-II). Detection rate did not vary significantly according to age, tumor location, differentiation, and TNM stage, except for gender. The follow-up testing of 40 postoperative patients with CRC returned negative results as their tumors had been surgically removed. The specificity of the sDNA test was 699/713 (98.0%), and unrelated cancers and diseases did not seem to interfere with the testing. The kappa value was 0.84, implying an excellent diagnostic consistency between the sDNA test and colonoscopy.
Conclusion: Noninvasive sDNA test using methylated SDC2 as the exclusive biomarker is a clinically viable and accurate CRC detection method.
Chinese clinical trial registry: Chi-CTR-TRC-1900026409, retrospectively registered on October 8, 2019; http://www.chictr.org.cn/edit.aspx?pid=43888&htm=4 .
Keywords: Advanced adenoma; Colorectal cancer; Methylation; Stool DNA test.
Conflict of interest statement
Xianglin Liu, Yan Qi, Feng Niu, Chunhua Chen, Xiaolin Wu, Xianshu Wang, and Hongzhi Zou are current employees from Creative Biosciences (Guangzhou) CO., Ltd. No potential conflicts of interests were disclosed by the other authors.
Figures


References
-
- International Agency for Research on Cancer. China source: Globocan 2018[R/OL]. [2019‐8‐1]. https://gco.iarc.fr/today/data/factsheets/populations/160‐china‐fact‐she....
-
- Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–573. doi: 10.1002/cncr.24760. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

