Cerebrospinal fluid findings in neurological diseases associated with COVID-19 and insights into mechanisms of disease development
- PMID: 33127503
- PMCID: PMC7591319
- DOI: 10.1016/j.ijid.2020.10.044
Cerebrospinal fluid findings in neurological diseases associated with COVID-19 and insights into mechanisms of disease development
Abstract
Objectives: To analyze the cerebrospinal fluid (CSF) of patients with SARS-CoV-2 infection and neurological manifestations to provide evidence for the understanding of mechanisms associated with central nervous system (CNS) involvement in COVID-19.
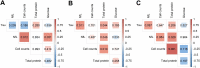
Methods: Patients (n = 58) were grouped according to their main neurological presentation: headache (n = 14); encephalopathy (n = 24); inflammatory neurological diseases, including meningoencephalitis (n = 4), acute myelitis (n = 3), meningitis (n = 2), acute disseminated encephalomyelitis (ADEM) (n = 2), encephalitis (n = 2), and neuromyelitis optica (n = 1); and Guillain-Barré syndrome (n = 6). Data regarding age, sex, cerebrovascular disease, and intracranial pressure were evaluated in combination with CSF profiles defined by cell counts, total protein and glucose levels, concentration of total Tau and neurofilament light chain (NfL) proteins, oligoclonal band patterns, and detection of SARS-CoV-2 RNA.
Results: CSF of patients with inflammatory neurological diseases was characterized by pleocytosis and elevated total protein and NfL levels. Patients with encephalopathy were mostly older men (mean age of 61.0 ± 17.6 years) with evidence of cerebrovascular disease. SARS-CoV-2 RNA in CSF was detected in 2 of 58 cases: a patient with refractory headache, and another patient who developed ADEM four days after onset of COVID-19 symptoms. Three patients presented intrathecal IgG synthesis, and four had identical oligoclonal bands in CSF and serum, indicating systemic inflammation.
Conclusion: Patients with neurological manifestations associated with COVID-19 had diverse CSF profiles, even within the same clinical condition. Our findings indicate a possible contribution of viral replication on triggering CNS infiltration by immune cells and the subsequent inflammation promoting neuronal injury.
Keywords: COVID-19; Cerebrospinal fluid; Neurofilament light protein; Oligoclonal bands; SARS-CoV-2; Total Tau protein.
Copyright © 2020 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- Araujo A.Q.C., Silva M.T.T., Araujo A.P.Q.C. Zika virus-associated neurological disorders: a review. Brain. 2016;139(August (Pt 8)):2122–2130. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

