Structure, mechanism, and regulation of mitochondrial DNA transcription initiation
- PMID: 33127643
- PMCID: PMC7939475
- DOI: 10.1074/jbc.REV120.011202
Structure, mechanism, and regulation of mitochondrial DNA transcription initiation
Abstract
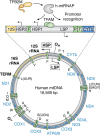
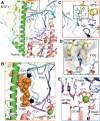
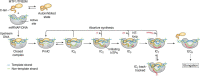
Mitochondria are specialized compartments that produce requisite ATP to fuel cellular functions and serve as centers of metabolite processing, cellular signaling, and apoptosis. To accomplish these roles, mitochondria rely on the genetic information in their small genome (mitochondrial DNA) and the nucleus. A growing appreciation for mitochondria's role in a myriad of human diseases, including inherited genetic disorders, degenerative diseases, inflammation, and cancer, has fueled the study of biochemical mechanisms that control mitochondrial function. The mitochondrial transcriptional machinery is different from nuclear machinery. The in vitro re-constituted transcriptional complexes of Saccharomyces cerevisiae (yeast) and humans, aided with high-resolution structures and biochemical characterizations, have provided a deeper understanding of the mechanism and regulation of mitochondrial DNA transcription. In this review, we will discuss recent advances in the structure and mechanism of mitochondrial transcription initiation. We will follow up with recent discoveries and formative findings regarding the regulatory events that control mitochondrial DNA transcription, focusing on those involved in cross-talk between the mitochondria and nucleus.
Keywords: DNA transcription; RNA polymerase; enzyme mechanism; enzyme structure; human mitochondrial RNA polymerase; mitochondria; mitochondrial DNA (mtDNA); mitochondrial DNA transcription; mitochondrial gene regulation; structure-function; transcription; transcription initiation factors; transcription regulation; yeast mitochondrial RNA polymerase.
© 2020 Basu et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Anderson S., Bankier A.T., Barrell B.G., de Bruijn M.H., Coulson A.R., Drouin J., Eperon I.C., Nierlich D.P., Roe B.A., Sanger F., Schreier P.H., Smith A.J., Staden R., Young I.G. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. 7219534. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

