The Notch Ligand Jagged1 Is Required for the Formation, Maintenance, and Survival of Hensen's Cells in the Mouse Cochlea
- PMID: 33127852
- PMCID: PMC7724135
- DOI: 10.1523/JNEUROSCI.1192-20.2020
The Notch Ligand Jagged1 Is Required for the Formation, Maintenance, and Survival of Hensen's Cells in the Mouse Cochlea
Abstract
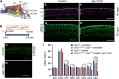
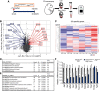
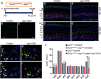
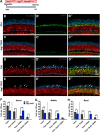
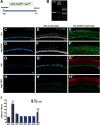
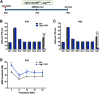
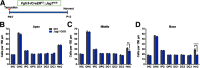
During cochlear development, the Notch ligand JAGGED 1 (JAG1) plays an important role in the specification of the prosensory region, which gives rise to sound-sensing hair cells and neighboring supporting cells (SCs). While JAG1's expression is maintained in SCs through adulthood, the function of JAG1 in SC development is unknown. Here, we demonstrate that JAG1 is essential for the formation and maintenance of Hensen's cells, a highly specialized SC subtype located at the edge of the auditory epithelium. Using Sox2CreERT2/+::Jag1loxP/loxP mice of both genders, we show that Jag1 deletion at the onset of differentiation, at embryonic day 14.5, disrupted Hensen's cell formation. Similar loss of Hensen's cells was observed when Jag1 was deleted after Hensen's cell formation at postnatal day (P) 0/P1 and fate-mapping analysis revealed that in the absence of Jag1, some Hensen's cells die, but others convert into neighboring Claudius cells. In support of a role for JAG1 in cell survival, genes involved in mitochondrial function and protein synthesis were downregulated in the sensory epithelium of P0 cochlea lacking Jag1 Finally, using Fgfr3-iCreERT2 ::Jag1loxP/loxP mice to delete Jag1 at P0, we observed a similar loss of Hensen's cells and found that adult Jag1 mutant mice have hearing deficits at the low-frequency range.SIGNIFICANCE STATEMENT Hensen's cells play an essential role in the development and homeostasis of the cochlea. Defects in the biophysical or functional properties of Hensen's cells have been linked to auditory dysfunction and hearing loss. Despite their importance, surprisingly little is known about the molecular mechanisms that guide their development. Morphologic and fate-mapping analyses in our study revealed that, in the absence of the Notch ligand JAGGED1, Hensen's cells died or converted into Claudius cells, which are specialized epithelium-like cells outside the sensory epithelium. Confirming a link between JAGGED1 and cell survival, transcriptional profiling showed that JAGGED1 maintains genes critical for mitochondrial function and tissue homeostasis. Finally, auditory phenotyping revealed that JAGGED1's function in supporting cells is necessary for low-frequency hearing.
Keywords: Claudius cells; Hensen's cells; Jagged1; Notch; cochlea development; supporting cells.
Copyright © 2020 the authors.
Figures
References
-
- Adam J, Myat A, Le Roux I, Eddison M, Henrique D, Ish-Horowicz D, Lewis J (1998) Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense-organ development. Development 125:4645–4654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
