Copper(I)-catalyzed asymmetric 1,6-conjugate allylation
- PMID: 33127903
- PMCID: PMC7603333
- DOI: 10.1038/s41467-020-19293-9
Copper(I)-catalyzed asymmetric 1,6-conjugate allylation
Abstract
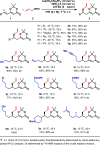
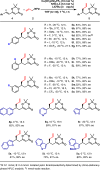
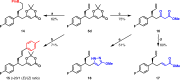
Catalytic asymmetric conjugate allylation of unsaturated carbonyl compounds is usually difficult to achieve, as 1,2-addition proceeds dominantly and high asymmetric induction is a challenging task. Herein, we disclose a copper(I)-NHC complex catalyzed asymmetric 1,6-conjugate allylation of 2,2-dimethyl-6-alkenyl-4H-1,3-dioxin-4-ones. The phenolic hydroxyl group in NHC ligands is found to be pivotal to obtain the desired products. Both aryl group and alkyl group at δ-position are well tolerated with the corresponding products generated in moderate to high yields and high enantioselectivity. Moreover, both 2-substituted and 3-substituted allylboronates serve as acceptable allylation reagents. At last, the synthetic utility of the products is demonstrated in several transformations by means of the versatile terminal olefin and dioxinone groups.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Copper(I)-Catalyzed Asymmetric Conjugate Addition of 1,4-Dienes to β-Substituted Alkenyl Azaarenes.J Am Chem Soc. 2023 Jan 25;145(3):1749-1758. doi: 10.1021/jacs.2c10688. Epub 2023 Jan 9. J Am Chem Soc. 2023. PMID: 36623207
-
Highly enantioselective Cu(I)-Tol-BINAP-catalyzed asymmetric conjugate addition of Grignard reagents to α,β-unsaturated esters.Chem Commun (Camb). 2010 Dec 14;46(46):8694-703. doi: 10.1039/c0cc03211e. Epub 2010 Oct 29. Chem Commun (Camb). 2010. PMID: 21038042
-
Copper(I)-Catalyzed Regioselective Asymmetric Addition of 1,4-Pentadiene to Ketones.J Am Chem Soc. 2021 Mar 31;143(12):4556-4562. doi: 10.1021/jacs.1c02084. Epub 2021 Mar 18. J Am Chem Soc. 2021. PMID: 33734679
-
Recent Progress of Substituted Allylboron Compounds in Catalytic Asymmetric Allylation Reactions.Chem Rec. 2025 Mar;25(3):e202400208. doi: 10.1002/tcr.202400208. Epub 2024 Dec 20. Chem Rec. 2025. PMID: 39707681 Review.
-
Asymmetric Synthesis of Alkyl Fluorides: Hydrogenation of Fluorinated Olefins.Angew Chem Int Ed Engl. 2019 Jul 1;58(27):9282-9287. doi: 10.1002/anie.201903954. Epub 2019 May 17. Angew Chem Int Ed Engl. 2019. PMID: 30995362 Review.
Cited by
-
Chiral oxazolidines acting as transient hydroxyalkyl-functionalized N-heterocyclic carbenes: an efficient route to air stable copper and gold complexes for asymmetric catalysis.Chem Sci. 2022 Jul 11;13(30):8773-8780. doi: 10.1039/d2sc02908a. eCollection 2022 Aug 4. Chem Sci. 2022. PMID: 35975143 Free PMC article.
-
Photochemical Organocatalytic Regio- and Enantioselective Conjugate Addition of Allyl Groups to Enals.Angew Chem Int Ed Engl. 2021 Dec 6;60(50):26373-26377. doi: 10.1002/anie.202111648. Epub 2021 Nov 10. Angew Chem Int Ed Engl. 2021. PMID: 34695283 Free PMC article.
-
Copper-catalysed asymmetric reductive cross-coupling of prochiral alkenes.Nat Commun. 2022 May 11;13(1):2570. doi: 10.1038/s41467-022-30286-8. Nat Commun. 2022. PMID: 35545634 Free PMC article.
-
Copper(I)-Catalyzed Asymmetric Conjugate 1,6-, 1,8-, and 1,10-Borylation.Angew Chem Int Ed Engl. 2021 Apr 19;60(17):9493-9499. doi: 10.1002/anie.202016081. Epub 2021 Mar 18. Angew Chem Int Ed Engl. 2021. PMID: 33543574 Free PMC article.
-
Chiral π-Cu(ii)-catalyzed site-, exo/endo-, and enantioselective dearomative (3 + 2) cycloadditions of isoquinolinium ylides with enamides, dienamides, and a trienamide.Chem Sci. 2024 Jun 11;15(28):10926-10934. doi: 10.1039/d4sc02946a. eCollection 2024 Jul 17. Chem Sci. 2024. PMID: 39027307 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

