Copper(I)-catalyzed asymmetric 1,6-conjugate allylation
- PMID: 33127903
- PMCID: PMC7603333
- DOI: 10.1038/s41467-020-19293-9
Copper(I)-catalyzed asymmetric 1,6-conjugate allylation
Abstract
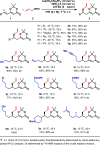
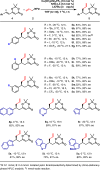
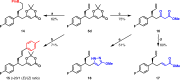
Catalytic asymmetric conjugate allylation of unsaturated carbonyl compounds is usually difficult to achieve, as 1,2-addition proceeds dominantly and high asymmetric induction is a challenging task. Herein, we disclose a copper(I)-NHC complex catalyzed asymmetric 1,6-conjugate allylation of 2,2-dimethyl-6-alkenyl-4H-1,3-dioxin-4-ones. The phenolic hydroxyl group in NHC ligands is found to be pivotal to obtain the desired products. Both aryl group and alkyl group at δ-position are well tolerated with the corresponding products generated in moderate to high yields and high enantioselectivity. Moreover, both 2-substituted and 3-substituted allylboronates serve as acceptable allylation reagents. At last, the synthetic utility of the products is demonstrated in several transformations by means of the versatile terminal olefin and dioxinone groups.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources

