PAX6 mutation alters circadian rhythm and β cell function in mice without affecting glucose tolerance
- PMID: 33127955
- PMCID: PMC7599253
- DOI: 10.1038/s42003-020-01337-x
PAX6 mutation alters circadian rhythm and β cell function in mice without affecting glucose tolerance
Abstract
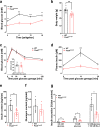
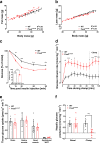
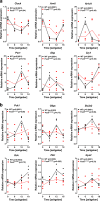
The transcription factor PAX6 is involved in the development of the eye and pancreatic islets, besides being associated with sleep-wake cycles. Here, we investigated a point mutation in the RED subdomain of PAX6, previously described in a human patient, to present a comprehensive study of a homozygous Pax6 mutation in the context of adult mammalian metabolism and circadian rhythm. Pax6Leca2 mice lack appropriate retinal structures for light perception and do not display normal daily rhythmic changes in energy metabolism. Despite β cell dysfunction and decreased insulin secretion, mutant mice have normal glucose tolerance. This is associated with reduced hepatic glucose production possibly due to altered circadian variation in expression of clock and metabolic genes, thereby evading hyperglycemia. Hence, our findings show that while the RED subdomain is important for β cell functional maturity, the Leca2 mutation impacts peripheral metabolism via loss of circadian rhythm, thus revealing pleiotropic effects of PAX6.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
The transcription factor Pax6 is required for pancreatic β cell identity, glucose-regulated ATP synthesis, and Ca2+ dynamics in adult mice.J Biol Chem. 2017 May 26;292(21):8892-8906. doi: 10.1074/jbc.M117.784629. Epub 2017 Apr 4. J Biol Chem. 2017. PMID: 28377501 Free PMC article.
-
High-fat diet induces early-onset diabetes in heterozygous Pax6 mutant mice.Diabetes Metab Res Rev. 2014 Sep;30(6):467-75. doi: 10.1002/dmrr.2572. Diabetes Metab Res Rev. 2014. PMID: 24925705
-
Pancreatic β cell enhancers regulate rhythmic transcription of genes controlling insulin secretion.Science. 2015 Nov 6;350(6261):aac4250. doi: 10.1126/science.aac4250. Science. 2015. PMID: 26542580 Free PMC article.
-
Circadian Transcription from Beta Cell Function to Diabetes Pathophysiology.J Biol Rhythms. 2016 Aug;31(4):323-36. doi: 10.1177/0748730416656949. J Biol Rhythms. 2016. PMID: 27440914 Free PMC article. Review.
-
Role of the clock gene Rev-erbα in metabolism and in the endocrine pancreas.Diabetes Obes Metab. 2015 Sep;17 Suppl 1:106-14. doi: 10.1111/dom.12522. Diabetes Obes Metab. 2015. PMID: 26332975 Review.
Cited by
-
Thirty Years' History since the Discovery of Pax6: From Central Nervous System Development to Neurodevelopmental Disorders.Int J Mol Sci. 2022 May 30;23(11):6115. doi: 10.3390/ijms23116115. Int J Mol Sci. 2022. PMID: 35682795 Free PMC article. Review.
-
Glucose Variability: How Does It Work?Int J Mol Sci. 2021 Jul 21;22(15):7783. doi: 10.3390/ijms22157783. Int J Mol Sci. 2021. PMID: 34360550 Free PMC article. Review.
-
Neural damage and neuroprotection with glaucoma development in aniridia.Curr Neurobiol. 2021;12(1):14-19. Curr Neurobiol. 2021. PMID: 38125639 Free PMC article. No abstract available.
-
Gene expression differences in the olfactory bulb associated with differential social interactions and olfactory deficits in Pax6 heterozygous mice.Biol Open. 2025 Feb 15;14(2):BIO061647. doi: 10.1242/bio.061647. Epub 2025 Feb 4. Biol Open. 2025. PMID: 39902612 Free PMC article.
-
ipRGCs Sensitive Blue Light Exposure Promotes the Robustness of Circadian and Neural Stem Cells in Sleep Deprived Conditions.Stem Cells Int. 2025 Jul 24;2025:8828183. doi: 10.1155/sci/8828183. eCollection 2025. Stem Cells Int. 2025. PMID: 40746442 Free PMC article.
References
-
- Stoykova A, Fritsch R, Walther C, Gruss P. Forebrain patterning defects in small eye mutant mice. Development. 1996;122:3453–3465. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

