Tissue-scale tensional homeostasis in skin regulates structure and physiological function
- PMID: 33127987
- PMCID: PMC7603398
- DOI: 10.1038/s42003-020-01365-7
Tissue-scale tensional homeostasis in skin regulates structure and physiological function
Abstract
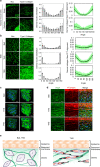
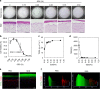
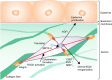
Tensional homeostasis is crucial for organ and tissue development, including the establishment of morphological and functional properties. Skin plays essential roles in waterproofing, cushioning and protecting deeper tissues by forming internal tension-distribution patterns, which involves aligning various cells, appendages and extracellular matrices (ECMs). The balance of traction force is thought to contribute to the formation of strong and pliable physical structures that maintain their integrity and flexibility. Here, by using a human skin equivalent (HSE), the horizontal tension-force balance of the dermal layer was found to clearly improve HSE characteristics, such as the physical relationship between cells and the ECM. The tension also promoted skin homeostasis through the activation of mechano-sensitive molecules such as ROCK and MRTF-A, and these results compared favourably to what was observed in tension-released models. Tension-induced HSE will contribute to analyze skin physiological functions regulated by tensional homeostasis as an alternative animal model.
Conflict of interest statement
T.T. is a supreme technical advisor at Organ Technologies Inc. This work was partially performed under the condition of an Invention Agreement between RIKEN, Rohto Pharmaceutical Co., Ltd., Meiji Seika Pharma Co., Ltd. and Organ Technologies Inc. The remaining authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

