Molecular specializations of deep cortical layer analogs in songbirds
- PMID: 33127988
- PMCID: PMC7599217
- DOI: 10.1038/s41598-020-75773-4
Molecular specializations of deep cortical layer analogs in songbirds
Abstract
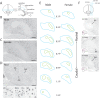
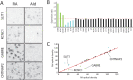
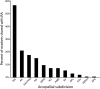
How the evolution of complex behavioral traits is associated with the emergence of novel brain pathways is largely unknown. Songbirds, like humans, learn vocalizations via tutor imitation and possess a specialized brain circuitry to support this behavior. In a comprehensive in situ hybridization effort, we show that the zebra finch vocal robust nucleus of the arcopallium (RA) shares numerous markers (e.g. SNCA, PVALB) with the adjacent dorsal intermediate arcopallium (AId), an avian analog of mammalian deep cortical layers with involvement in motor function. We also identify markers truly unique to RA and thus likely linked to modulation of vocal motor function (e.g. KCNC1, GABRE), including a subset of the known shared markers between RA and human laryngeal motor cortex (e.g. SLIT1, RTN4R, LINGO1, PLXNC1). The data provide novel insights into molecular features unique to vocal learning circuits, and lend support for the motor theory for vocal learning origin.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Zeigler HP, Marler P. In: Behavioral Neurobiology of Birdsong. Zeigler HP, Marler P, editors. New York: New York Academy of Sciences; 2004.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

