Th22 response induced by Mycobacterium tuberculosis strains is closely related to severity of pulmonary lesions and bacillary load in patients with multi-drug-resistant tuberculosis
- PMID: 33128773
- PMCID: PMC7806416
- DOI: 10.1111/cei.13544
Th22 response induced by Mycobacterium tuberculosis strains is closely related to severity of pulmonary lesions and bacillary load in patients with multi-drug-resistant tuberculosis
Abstract
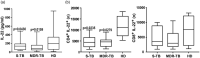
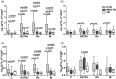
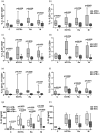
The role of interleukin-22 (IL-22) in the pathogenesis or tissue repair in human tuberculosis (TB) remains to be established. Here, we aimed to explore the ex-vivo and in-vitro T helper 22 (Th22) response in TB patients and healthy donors (HD) induced by different local multi-drug-resistant (MDR) Mvcobacterium tuberculosis (Mtb) strains. For this purpose, peripheral blood mononuclear cells from drug-susceptible (S-TB) MDR-TB patients and HD were stimulated with local MDR strains and the laboratory strain H37Rv. IL-22 and IL-17 expression and senescent status were assessed in CD4+ and CD8+ cells by flow cytometry, while IL-22 amount was measured in plasma and culture supernatants by enzyme-linked immunosorbent assay (ELISA). We found lower IL-22 amounts in plasma from TB patients than HD, together with a decrease in the number of circulating T cells expressing IL-22. In a similar manner, all Mtb strains enhanced IL-22 secretion and expanded IL-22+ cells within CD4+ and CD8+ subsets, being the highest levels detected in S-TB patients. In MDR-TB, low systemic and Mtb-induced Th22 responses associated with high sputum bacillary load and bilateralism of lung lesions, suggesting that Th22 response could be influencing the ability of MDR-TB patients to control bacillary growth and tissue damage. In addition, in MDR-TB patients we observed that the higher the percentage of IL-22+ cells, the lower the proportion of programmed cell death 1 (PD-1)+ or CD57+ T cells. Furthermore, the highest proportion of senescent T cells was associated with severe lung lesions and bacillary load. Thus, T cell senescence would markedly influence Th22 response mounted by MDR-TB patients.
Keywords: IL-22; Mycobacterium tuberculosis strains; Th22 response; multi-drug-resistant tuberculosis.
© 2020 British Society for Immunology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Wolk K, Sabat R. Interleukin‐22: a novel T‐ and NK‐cell derived cytokine that regulates the biology of tissue cells. Cytokine Growth Factor Rev 2006; 17:367–80. - PubMed
-
- Sonnenberg GF, Fouser LA, Artis D. Functional biology of the IL‐22‐IL‐22R pathway in regulating immunity and inflammation at barrier surfaces. Adv Immunol 2010; 107:1–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

