Minimally invasive esophageal sponge cytology sampling is feasible in a Tanzanian community setting
- PMID: 33128785
- PMCID: PMC7839767
- DOI: 10.1002/ijc.33366
Minimally invasive esophageal sponge cytology sampling is feasible in a Tanzanian community setting
Abstract
Esophageal sponge cytology is an endoscopy alternative well accepted by patients with extensive data for accuracy in the context of adenocarcinoma. Few studies have assessed its feasibility in asymptomatic community members, and fewer still in East Africa, where esophageal squamous cell carcinoma (ESCC) rates are high. We aimed to assess the feasibility of a capsule-based diagnosis of esophageal squamous dysplasia (ESD), an ESCC precursor, which may benefit epidemiological and early detection research. We collected Cytosponge collections in 102 asymptomatic adults from Kilimanjaro, Tanzania. Uptake, acceptability and safety were assessed. Participants scored acceptability immediately following the procedure and 7 days later on a scale of 0 (least) to 10 (most acceptable). Slides from paraffin-embedded cell clots were read by two pathologists for ESD and other pathologies. All participants (52 men, 50 women, aged 30-77) swallowed the device at first attempt, 100 (98%) of which gave slides of adequate cellularity. Acceptability scores were 10 (53%), 9 (24%), 8 (21%), 7 (2%) and 6 (1%), with no differences by age, sex or time of asking. Cytological findings were esophageal inflammation (4%), atypical squamous cells of uncertain significance (1%), low-grade dysplasia (1%), gastritis (22%) and suspected intestinal metaplasia (6%). Setting-specific logistical and ethical considerations of study implementation are discussed. We demonstrate the safety, acceptability and feasibility of Cytosponge sampling in this setting, paving the way for innovative etiology and early-detection research. Targeted sampling strategies and biomarker development will underpin the success of such initiatives. The study protocol is registered on ClinicalTrials.gov (NCT04090554).
Keywords: Africa; Cytosponge; esophageal cancer; squamous dysplasia.
© 2020 International Agency for Research on Cancer (IARC/WHO); licensed by John Wiley & Sons Ltd on behalf of Union for International Cancer Control.
Conflict of interest statement
The Cytosponge device was designed by RCF and the MRC licensed the technology to Covidien GI Solutions, now part of Medtronic. Medtronic have had no influence on the design, conduct or analysis of this study. RCF and MOD are named inventors on patents pertaining to the Cytosponge and related assays. RCF and MoD are shareholders and consultants for Cyted Ltd. All other authors declare no conflicts of interest.
Figures
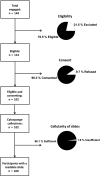
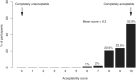

Comment in
-
Esophageal sponge sampling: A promising approach for early detection of esophageal cancer in Africa's high-incidence region.Int J Cancer. 2021 Apr 1;148(7):1546-1547. doi: 10.1002/ijc.33372. Epub 2020 Nov 21. Int J Cancer. 2021. PMID: 33128772 No abstract available.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. - PubMed
-
- Arnold M, Ferlay J, van Berge Henegouwen MI, Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020;69:1564‐1571. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

