A Bayesian averted infection framework for PrEP trials with low numbers of HIV infections: application to the results of the DISCOVER trial
- PMID: 33128906
- PMCID: PMC7664988
- DOI: 10.1016/S2352-3018(20)30192-2
A Bayesian averted infection framework for PrEP trials with low numbers of HIV infections: application to the results of the DISCOVER trial
Abstract
Trials of candidate agents for HIV pre-exposure prophylaxis (PrEP) might randomly assign participants to be given a new PrEP agent or oral coformulated tenofovir disoproxil fumarate plus emtricitabine. This design presents unique challenges in interpretation. First, with two active arms, HIV incidence might be low. Second, the effectiveness of tenofovir disoproxil fumarate plus emtricitabine varies across populations; thus, similar HIV incidence between groups could be consistent with a wide range of effectiveness for the new PrEP. We propose a two-part approach to trial results. First, we use Bayesian methods to incorporate assumptions about the background incidence of HIV in the trial in the absence of PrEP, possibly augmented by external data. On the basis of the estimated background incidence, we estimate and compare the number of averted (or prevented) HIV infections in each of the two trial groups, calculating the averted infections ratio. We apply these methods to a completed trial of tenofovir alafenamide plus emtricitabine for PrEP. Our framework shows that leveraging external information to estimate averted infections and the averted infections ratio enhances the efficiency and interpretation of active-controlled PrEP trials.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
DTD reports personal fees from ViiV Healthcare and Gilead Sciences, outside of the submitted work. DVG has accepted fees from Gilead Sciences and Merck.
Figures


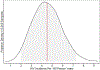
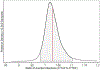
References
-
- Sugarman J, Celum CL, Donnell D, Mayer KH. Ethical considerations for new HIV prevention trials. Lancet HIV. 2019; 6:e489–91. - PubMed
-
- Donnell D. Current and future challenges in trial design for pre-exposure prophylaxis in HIV prevention. Stat Comm Inf Dis. 2019;11(1).
-
- Safety and efficacy of emtricitabine and tenofovir alafenamide (F/TAF) Fixed-dose combination once daily for pre-exposure prophylaxis in men and transgender women who have sex with men and are at risk of HIV-1 infection (DISCOVER). https://clinicaltrials.gov/ct2/show/NCT02842086 Retrieved on 30 April 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

