Incidence of influenza during pregnancy and association with pregnancy and perinatal outcomes in three middle-income countries: a multisite prospective longitudinal cohort study
- PMID: 33129424
- PMCID: PMC10563867
- DOI: 10.1016/S1473-3099(20)30592-2
Incidence of influenza during pregnancy and association with pregnancy and perinatal outcomes in three middle-income countries: a multisite prospective longitudinal cohort study
Erratum in
-
Correction to Lancet Infect Dis 2020; published online Oct 29. https://doi.org/10.1016/S1473-3099(20)30592-2.Lancet Infect Dis. 2021 Jan;21(1):e1. doi: 10.1016/S1473-3099(20)30864-1. Epub 2020 Nov 6. Lancet Infect Dis. 2021. PMID: 33166491 No abstract available.
Abstract
Background: Influenza vaccination during pregnancy prevents influenza among women and their infants but remains underused among pregnant women. We aimed to quantify the risk of antenatal influenza and examine its association with perinatal outcomes.
Methods: We did a prospective cohort study in pregnant women in India, Peru, and Thailand. Before the 2017 and 2018 influenza seasons, we enrolled pregnant women aged 18 years or older with expected delivery dates 8 weeks or more after the season started. We contacted women twice weekly until the end of pregnancy to identify illnesses with symptoms of myalgia, cough, runny nose or nasal congestion, sore throat, or difficulty breathing and collected mid-turbinate nasal swabs from symptomatic women for influenza real-time RT-PCR testing. We assessed the association of antenatal influenza with preterm birth, late pregnancy loss (≥13 weeks gestation), small for gestational age (SGA), and birthweight of term singleton infants using Cox proportional hazards models or generalised linear models to adjust for potential confounders.
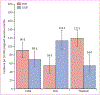
Findings: Between March 13, 2017, and Aug 3, 2018, we enrolled 11 277 women with a median age of 26 years (IQR 23-31) and gestational age of 19 weeks (14-24). 1474 (13%) received influenza vaccines. 310 participants (3%) had influenza (270 [87%] influenza A and 40 [13%] influenza B). Influenza incidences weighted by the population of women of childbearing age in each study country were 88·7 per 10 000 pregnant woman-months (95% CI 68·6 to 114·8) during the 2017 season and 69·6 per 10 000 pregnant woman-months (53·8 to 90·2) during the 2018 season. Antenatal influenza was not associated with preterm birth (adjusted hazard ratio [aHR] 1·4, 95% CI 0·9 to 2·0; p=0·096) or having an SGA infant (adjusted relative risk 1·0, 95% CI 0·8 to 1·3, p=0·97), but was associated with late pregnancy loss (aHR 10·7, 95% CI 4·3 to 27·0; p<0·0001) and reduction in mean birthweight of term, singleton infants (-55·3 g, 95% CI -109·3 to -1·4; p=0·0445).
Interpretation: Women had a 0·7-0·9% risk of influenza per month of pregnancy during the influenza season, and antenatal influenza was associated with increased risk for some adverse pregnancy outcomes. These findings support the added value of antenatal influenza vaccination to improve perinatal outcomes.
Funding: US Centers for Disease Control and Prevention.
Translations: For the Thai, Hindi, Marathi and Spanish translations of the abstract see Supplementary Materials section.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
We declare no competing interests.
Figures
Comment in
-
Time to prioritise influenza vaccination in pregnancy.Lancet Infect Dis. 2021 Jan;21(1):8-10. doi: 10.1016/S1473-3099(20)30679-4. Epub 2020 Oct 29. Lancet Infect Dis. 2021. PMID: 33129423 No abstract available.
References
-
- WHO. Vaccines against influenza WHO position paper—November 2012. Wkly Epidemiol Rec 2012; 87: 461–76. - PubMed
-
- Madhi SA, Cutland CL, Kuwanda L, et al. Influenza vaccination of pregnant women and protection of their infants. N Engl J Med 2014; 371: 918–31. - PubMed
-
- Thompson MG, Kwong JC, Regan AK, et al. Influenza vaccine effectiveness in preventing influenza-associated hospitalizations during pregnancy: a multi-country retrospective test negative design study, 2010–2016. Clin Infect Dis 2019; 68: 1444–53. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

