PD-1 Signaling Promotes Tumor-Infiltrating Myeloid-Derived Suppressor Cells and Gastric Tumorigenesis in Mice
- PMID: 33129844
- PMCID: PMC7878361
- DOI: 10.1053/j.gastro.2020.10.036
PD-1 Signaling Promotes Tumor-Infiltrating Myeloid-Derived Suppressor Cells and Gastric Tumorigenesis in Mice
Abstract
Background & aims: Immune checkpoint inhibitors have limited efficacy in many tumors. We investigated mechanisms of tumor resistance to inhibitors of programmed cell death-1 (PDCD1, also called PD-1) in mice with gastric cancer, and the role of its ligand, PD-L1.
Methods: Gastrin-deficient mice were given N-methyl-N-nitrosourea (MNU) in drinking water along with Helicobacter felis to induce gastric tumor formation; we also performed studies with H/K-ATPase-hIL1B mice, which develop spontaneous gastric tumors at the antral-corpus junction and have parietal cells that constitutively secrete interleukin 1B. Mice were given injections of an antibody against PD-1 or an isotype control before tumors developed, or anti-PD-1 and 5-fluorouracil and oxaliplatin, or an antibody against lymphocyte antigen 6 complex locus G (also called Gr-1), which depletes myeloid-derived suppressor cells [MDSCs]), after tumors developed. We generated knock-in mice that express PD-L1 specifically in the gastric epithelium or myeloid lineage.
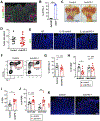
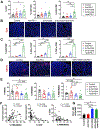
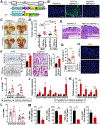
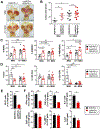
Results: When given to gastrin-deficient mice before tumors grew, anti-PD-1 significantly reduced tumor size and increased tumor infiltration by T cells. However, anti-PD-1 alone did not have significant effects on established tumors in these mice. Neither early nor late anti-PD-1 administration reduced tumor growth in the presence of MDSCs in H/K-ATPase-hIL-1β mice. The combination of 5-fluorouracil and oxaliplatin reduced MDSCs, increased numbers of intra-tumor CD8+ T cells, and increased the response of tumors to anti-PD-1; however, this resulted in increased tumor expression of PD-L1. Expression of PD-L1 by tumor or immune cells increased gastric tumorigenesis in mice given MNU. Mice with gastric epithelial cells that expressed PD-L1 did not develop spontaneous tumors, but they developed more and larger tumors after administration of MNU and H felis, with accumulation of MDSCs.
Conclusions: In mouse models of gastric cancer, 5-fluorouracil and oxaliplatin reduce numbers of MDSCs to increase the effects of anti-PD-1, which promotes tumor infiltration by CD8+ T cells. However, these chemotherapeutic agents also induce expression of PD-L1 by tumor cells. Expression of PD-L1 by gastric epithelial cells increases tumorigenesis in response to MNU and H felis, and accumulation of MDSCs, which promote tumor progression. The timing and site of PD-L1 expression is therefore important in gastric tumorigenesis and should be considered in design of therapeutic regimens.
Keywords: Immunosuppression; Mouse Model; Resistance; Stomach Cancer.
Copyright © 2021 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures



Comment in
-
Targeting the Myeloid-Derived Suppressor Cell Compartment for Inducing Responsiveness to Immune Checkpoint Blockade Is Best Limited to Specific Subtypes of Gastric Cancers.Gastroenterology. 2021 Aug;161(2):727. doi: 10.1053/j.gastro.2021.03.047. Epub 2021 Mar 31. Gastroenterology. 2021. PMID: 33798523 No abstract available.
-
Reply.Gastroenterology. 2021 Aug;161(2):727-728. doi: 10.1053/j.gastro.2021.04.027. Epub 2021 Apr 19. Gastroenterology. 2021. PMID: 33887218 No abstract available.
References
-
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. - PubMed
-
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2016, National Cancer Institute; Bethesda, MD, https://seer.cancer.gov/csr/1975_2016/, based on November 2018 SEER data submission, posted to the SEER web site, April 2019. 2019.
-
- Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v38–v49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

