The dynamic regulation of appetitive behavior through lateral hypothalamic orexin and melanin concentrating hormone expressing cells
- PMID: 33130035
- PMCID: PMC8041470
- DOI: 10.1016/j.physbeh.2020.113234
The dynamic regulation of appetitive behavior through lateral hypothalamic orexin and melanin concentrating hormone expressing cells
Abstract
The lateral hypothalamic area (LHA) is a heterogeneous brain structure extensively studied for its potent role in regulating energy balance. The anatomical and molecular diversity of the LHA permits the orchestration of responses to energy sensing cues from the brain and periphery. Two of the primary cell populations within the LHA associated with integration of this information are Orexin (ORX) and Melanin Concentrating Hormone (MCH). While both of these non-overlapping populations exhibit orexigenic properties, the activities of these two systems support feeding behavior through contrasting mechanisms. We describe the anatomical and functional properties as well as interaction with other neuropeptides and brain reward and hedonic systems. Specific outputs relating to arousal, food seeking, feeding, and metabolism are coordinated through these mechanisms. We then discuss how both the ORX and MCH systems harmonize in a divergent yet overall cooperative manner to orchestrate feeding behavior through transitions between various appetitive states, and thus offer novel insights into LHA allostatic control of appetite.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures

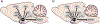

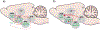
Similar articles
-
Neuropeptide Y cells represent a distinct glucose-sensing population in the lateral hypothalamus.Endocrinology. 2011 Nov;152(11):4046-52. doi: 10.1210/en.2011-1307. Epub 2011 Sep 13. Endocrinology. 2011. PMID: 21914773 Free PMC article.
-
Neurochemical Heterogeneity Among Lateral Hypothalamic Hypocretin/Orexin and Melanin-Concentrating Hormone Neurons Identified Through Single-Cell Gene Expression Analysis.eNeuro. 2017 Sep 22;4(5):ENEURO.0013-17.2017. doi: 10.1523/ENEURO.0013-17.2017. eCollection 2017 Sep-Oct. eNeuro. 2017. PMID: 28966976 Free PMC article.
-
Striatal medium spiny neurons terminate in a distinct region in the lateral hypothalamic area and do not directly innervate orexin/hypocretin- or melanin-concentrating hormone-containing neurons.J Neurosci. 2007 Jun 27;27(26):6948-55. doi: 10.1523/JNEUROSCI.0514-07.2007. J Neurosci. 2007. PMID: 17596443 Free PMC article.
-
Hubs and spokes of the lateral hypothalamus: cell types, circuits and behaviour.J Physiol. 2016 Nov 15;594(22):6443-6462. doi: 10.1113/JP271946. Epub 2016 Jul 19. J Physiol. 2016. PMID: 27302606 Free PMC article. Review.
-
Lateral hypothalamus as a sensor-regulator in respiratory and metabolic control.Physiol Behav. 2013 Sep 10;121:117-24. doi: 10.1016/j.physbeh.2013.03.023. Epub 2013 Apr 3. Physiol Behav. 2013. PMID: 23562864 Free PMC article. Review.
Cited by
-
Male and female mice may respectively form stronger social aversive memories with same and different sex conspecifics.bioRxiv [Preprint]. 2025 Jun 12:2024.08.12.607663. doi: 10.1101/2024.08.12.607663. bioRxiv. 2025. PMID: 39185229 Free PMC article. Preprint.
-
Neuropeptides Modulate Feeding via the Dopamine Reward Pathway.Neurochem Res. 2023 Sep;48(9):2622-2643. doi: 10.1007/s11064-023-03954-4. Epub 2023 May 26. Neurochem Res. 2023. PMID: 37233918 Review.
-
Hypothalamic GABRA5-positive neurons control obesity via astrocytic GABA.Nat Metab. 2023 Sep;5(9):1506-1525. doi: 10.1038/s42255-023-00877-w. Epub 2023 Aug 31. Nat Metab. 2023. PMID: 37653043
-
Efferent and Afferent Connections of Neuropeptide Y Neurons in the Nucleus Accumbens of Mice.Front Neuroanat. 2021 Sep 10;15:741868. doi: 10.3389/fnana.2021.741868. eCollection 2021. Front Neuroanat. 2021. PMID: 34566585 Free PMC article.
-
Physiological Role of Orexinergic System for Health.Int J Environ Res Public Health. 2022 Jul 8;19(14):8353. doi: 10.3390/ijerph19148353. Int J Environ Res Public Health. 2022. PMID: 35886210 Free PMC article. Review.
References
-
- Johnson AW. Eating beyond metabolic need: how environmental cues influence feeding behavior. Trends Neurosci. 2013;36:101–9. - PubMed
-
- Myers MG, Cowley MA, & Münzberg H Mechanisms of leptin action and leptinresistance. Annu. Rev. Physiol, 2008; 70, 537–556. - PubMed
-
- Williams LM. Hypothalamic dysfunction in obesity. Proc Nutr Soc. 2012;71:521–33. - PubMed
-
- Brobeck JR. Mechanism of the development of obesity in animals with hypothalamic lesions. Physiol Rev. 1946;26:541–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

