Current developments of targeting the p53 signaling pathway for cancer treatment
- PMID: 33130194
- PMCID: PMC7969395
- DOI: 10.1016/j.pharmthera.2020.107720
Current developments of targeting the p53 signaling pathway for cancer treatment
Abstract
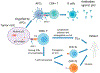
p53 is one of the most well-studied tumor suppressors. It is mutated or deleted in half of all cancers. In the other half carrying wild type p53, the p53 signaling pathway is disrupted by abnormalities of other components in the pathway. Due to its paramount role in tumor suppression, p53 has attracted great interest in drug development as any clinically successful therapeutic agent to target the p53 pathway will save millions of lives. However, designing therapeutics targeting the pathway has been extremely challenging, despite more than forty years of research. This review will summarize past and current efforts of developing p53-based gene therapy and targeted therapies for cancer treatment. In addition, the current efforts of exploiting the immunogenicity of p53 protein for cancer immunotherapy will be reviewed. Challenges and future directions for targeting the p53 pathway will be discussed.
Keywords: Cancer; Cancer therapy; Gain of function; Immunotherapy; Tumor suppressor; p53.
Published by Elsevier Inc.
Conflict of interest statement
Conflict of Interest
The author has no conflict of interest.
Figures


References
-
- Lane DP & Crawford LV T antigen is bound to a host protein in SV40-transformed cells. Nature 278, 261–263 (1979). - PubMed
-
- Linzer DI & Levine AJ Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell 17, 43–52 (1979). - PubMed
-
- Melero JA, Stitt DT, Mangel WF & Carroll RB Identification of new polypeptide species (48-55K) immunoprecipitable by antiserum to purified large T antigen and present in SV40-infected and -transformed cells. Virology 93, 466–480 (1979). - PubMed
-
- Smith AE, Smith R & Paucha E Characterization of different tumor antigens present in cells transformed by simian virus 40. Cell 18, 335–346 (1979). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

