A new role for matrix metalloproteinase-3 in the NGF metabolic pathway: Proteolysis of mature NGF and sex-specific differences in the continuum of Alzheimer's pathology
- PMID: 33130223
- PMCID: PMC7856186
- DOI: 10.1016/j.nbd.2020.105150
A new role for matrix metalloproteinase-3 in the NGF metabolic pathway: Proteolysis of mature NGF and sex-specific differences in the continuum of Alzheimer's pathology
Abstract
Matrix metalloproteinase-3 (MMP-3) has been associated with risk of Alzheimer's disease (AD). In this study we introduce a novel role for MMP-3 in degrading nerve growth factor (NGF) in vivo and examine its mRNA and protein expression across the continuum of AD pathology. We provide evidence that MMP-3 participates in the degradation of mature NGF in vitro and in vivo and that it is secreted from the rat cerebral cortex in an activity-dependent manner. We show that cortical MMP-3 is upregulated in the McGill-R-Thy1-APP transgenic rat model of AD-like amyloidosis. A similar upregulation was found in AD and MCI brains as well as in cognitively normal individuals with elevated amyloid deposition. We also observed that frontal cortex MMP-3 protein levels are higher in males. MMP-3 protein correlated with more AD neuropathology, markers of NGF metabolism, and lower cognitive scores in males but not in females. These results suggest that MMP-3 upregulation in AD might contribute to NGF dysmetabolism, and therefore to cholinergic atrophy and cognitive deficits, in a sex-specific manner. MMP-3 should be further investigated as a biomarker candidate or as a therapeutic target in AD.
Keywords: Alzheimer's disease; Basal forebrain cholinergic neurons; Matrix metalloproteinase-3; NGF metabolic pathway; Nerve growth factor; proNGF.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest
The authors have no conflict of interest to report.
Figures
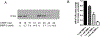
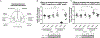
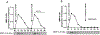

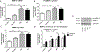

Similar articles
-
Differential deregulation of NGF and BDNF neurotrophins in a transgenic rat model of Alzheimer's disease.Neurobiol Dis. 2017 Dec;108:307-323. doi: 10.1016/j.nbd.2017.08.019. Epub 2017 Sep 1. Neurobiol Dis. 2017. PMID: 28865749
-
The human brain NGF metabolic pathway is impaired in the pre-clinical and clinical continuum of Alzheimers disease.Mol Psychiatry. 2021 Oct;26(10):6023-6037. doi: 10.1038/s41380-020-0797-2. Epub 2020 Jun 2. Mol Psychiatry. 2021. PMID: 32488129 Free PMC article.
-
Nerve growth factor (NGF) pathway biomarkers in Down syndrome prior to and after the onset of clinical Alzheimer's disease: A paired CSF and plasma study.Alzheimers Dement. 2021 Apr;17(4):605-617. doi: 10.1002/alz.12229. Epub 2020 Nov 23. Alzheimers Dement. 2021. PMID: 33226181 Free PMC article.
-
The Nerve Growth Factor Metabolic Pathway Dysregulation as Cause of Alzheimer's Cholinergic Atrophy.Cells. 2021 Dec 22;11(1):16. doi: 10.3390/cells11010016. Cells. 2021. PMID: 35011577 Free PMC article. Review.
-
NGF-cholinergic dependency in brain aging, MCI and Alzheimer's disease.Curr Alzheimer Res. 2007 Sep;4(4):351-8. doi: 10.2174/156720507781788774. Curr Alzheimer Res. 2007. PMID: 17908036 Review.
Cited by
-
Sex-Related Differences of Matrix Metalloproteinases (MMPs): New Perspectives for These Biomarkers in Cardiovascular and Neurological Diseases.J Pers Med. 2022 Jul 22;12(8):1196. doi: 10.3390/jpm12081196. J Pers Med. 2022. PMID: 35893290 Free PMC article. Review.
-
The Link Between Matrix Metalloproteinases and Alzheimer's Disease Pathophysiology.Mol Neurobiol. 2025 Jan;62(1):885-899. doi: 10.1007/s12035-024-04315-0. Epub 2024 Jun 27. Mol Neurobiol. 2025. PMID: 38935232 Free PMC article. Review.
-
Nerve growth factor precursor alterations in neuron-derived extracellular vesicles from individuals with Down syndrome along the Alzheimer's disease continuum.Alzheimers Dement. 2025 Apr;21(4):e70137. doi: 10.1002/alz.70137. Alzheimers Dement. 2025. PMID: 40257051 Free PMC article.
-
Novel targets for the treatment and prevention of Alzheimer's disease in the European population, inspiration from amyloid beta and tau protein.Heliyon. 2024 Oct 5;10(20):e39013. doi: 10.1016/j.heliyon.2024.e39013. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39492919 Free PMC article.
-
Tau truncation in the pathogenesis of Alzheimer's disease: a narrative review.Neural Regen Res. 2024 Jun 1;19(6):1221-1232. doi: 10.4103/1673-5374.385853. Epub 2023 Sep 22. Neural Regen Res. 2024. PMID: 37905868 Free PMC article.
References
-
- Almodóvar R, et al., 2014. Association of biomarkers of inflammation, cartilage and bone turnover with gender, disease activity, radiological damage and sacroiliitis by magnetic resonance imaging in patients with early spondyloarthritis. Clinical rheumatology. 33, 237–241. - PubMed
-
- Arends S, et al., 2011. Serum MMP-3 level as a biomarker for monitoring and predicting response to etanercept treatment in ankylosing spondylitis. 38, 1644–1650. - PubMed
-
- Baig S, et al., 2008. MMP-2,-3 and-9 levels and activity are not related to Aβ load in the frontal cortex in Alzheimer’s disease. Neuropathology and applied neurobiology. 34, 205–215. - PubMed
-
- Bartus RT, et al., 1982. The cholinergic hypothesis of geriatric memory dysfunction. Science. 217, 408–414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

