Effect of Intensive Urate Lowering With Combined Verinurad and Febuxostat on Albuminuria in Patients With Type 2 Diabetes: A Randomized Trial
- PMID: 33130235
- PMCID: PMC8045740
- DOI: 10.1053/j.ajkd.2020.09.009
Effect of Intensive Urate Lowering With Combined Verinurad and Febuxostat on Albuminuria in Patients With Type 2 Diabetes: A Randomized Trial
Abstract
Rationale & objective: Hyperuricemia has been implicated in the development and progression of chronic kidney disease. Verinurad is a novel, potent, specific urate reabsorption inhibitor. We evaluated the effects on albuminuria of intensive urate-lowering therapy with verinurad combined with the xanthine oxidase inhibitor febuxostat in patients with hyperuricemia and type 2 diabetes mellitus (T2DM).
Study design: Phase 2, multicenter, prospective, randomized, double-blind, parallel-group, placebo-controlled trial.
Setting & participants: Patients 18 years or older with hyperuricemia, albuminuria, and T2DM.
Intervention: Patients randomly assigned 1:1 to verinurad (9mg) plus febuxostat (80mg) or matched placebo once daily for 24 weeks.
Outcomes: The primary end point was change in urinary albumin-creatinine ratio (UACR) from baseline after 12 weeks' treatment. Secondary end points included safety and tolerability and effect on glomerular filtration.
Results: 60 patients were enrolled (n=32, verinurad and febuxostat; n=28, placebo). UACRs after treatment with verinurad plus febuxostat were lower than after placebo at 1, 12, and 24 weeks: -38.6% (90% CI, -60.9% to-3.6%), -39.4% (90% CI, -61.8% to-3.8%), and-49.3% (90% CI, -68.2% to-19.0%), respectively. Serum urate levels after treatment with verinurad plus febuxostat were 59.6% and 63.7% lower than after placebo at 12 and 24 weeks, respectively. No clinically meaningful changes were observed in estimated glomerular filtration rate or serum creatinine or serum cystatin C concentrations. Verinurad plus febuxostat was well tolerated.
Limitations: Sample size and study duration were insufficient to evaluate definitive effects of verinurad plus febuxostat on UACR and glomerular filtration. Generalizability was limited by exclusion of patients with stages 4 and 5 chronic kidney disease.
Conclusions: Verinurad plus febuxostat reduced albuminuria and lowered serum urate concentrations in patients with T2DM, albuminuria, and hyperuricemia. Definitive assessment of their combined impact on preservation of kidney function awaits larger clinical studies.
Funding: This study was supported by AstraZeneca.
Trial registration: Registered at ClinicalTrials.gov with study number NCT03118739.
Keywords: Albuminuria; cardiovascular outcomes; chronic kidney disease (CKD); febuxostat; hyperuricemia; randomized controlled trial (RCT); renal function; safety; type 2 diabetes; urate lowering; urinary albumin-creatinine ratio (UACR); verinurad.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
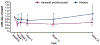


Comment in
-
Urate Lowering With Combination Therapy in CKD: Reason for Optimism or Einstein's Definition of Insanity?Am J Kidney Dis. 2021 Apr;77(4):478-480. doi: 10.1053/j.ajkd.2020.11.007. Epub 2021 Feb 7. Am J Kidney Dis. 2021. PMID: 33568321 No abstract available.
-
In Reply to 'Verinurad/Febuxostat and Nephrotoxicity'.Am J Kidney Dis. 2021 Sep;78(3):468-469. doi: 10.1053/j.ajkd.2021.05.009. Epub 2021 Jun 11. Am J Kidney Dis. 2021. PMID: 34126126 No abstract available.
-
Verinurad/Febuxostat and Nephrotoxicity.Am J Kidney Dis. 2021 Sep;78(3):468. doi: 10.1053/j.ajkd.2021.03.029. Epub 2021 Jun 11. Am J Kidney Dis. 2021. PMID: 34126127 No abstract available.
References
-
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. - PMC - PubMed
-
- Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2020;75(1S1):A6–A7. - PubMed

