Xenogeneic-free generation of vascular smooth muscle cells from human induced pluripotent stem cells for vascular tissue engineering
- PMID: 33130306
- PMCID: PMC8168373
- DOI: 10.1016/j.actbio.2020.10.042
Xenogeneic-free generation of vascular smooth muscle cells from human induced pluripotent stem cells for vascular tissue engineering
Abstract
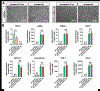
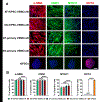
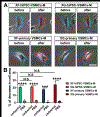
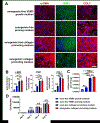
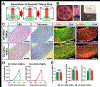
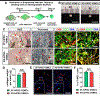
Development of mechanically advanced tissue-engineered vascular grafts (TEVGs) from human induced pluripotent stem cell (hiPSC)-derived vascular smooth muscle cells (hiPSC-VSMCs) offers an innovative approach to replace or bypass diseased blood vessels. To move current hiPSC-TEVGs toward clinical application, it is essential to obtain hiPSC-VSMC-derived tissues under xenogeneic-free conditions, meaning without the use of any animal-derived reagents. Many approaches in VSMC differentiation of hiPSCs have been reported, although a xenogeneic-free method for generating hiPSC-VSMCs suitable for vascular tissue engineering has yet to be established. Based on our previously established standard method of xenogeneic VSMC differentiation, we have replaced all animal-derived reagents with functional counterparts of human origin and successfully derived functional xenogeneic-free hiPSC-VSMCs (XF-hiPSC-VSMCs). Next, our group developed tissue rings via cellular self-assembly from XF-hiPSC-VSMCs, which exhibited comparable mechanical strength to those developed from xenogeneic hiPSC-VSMCs. Moreover, by seeding XF-hiPSC-VSMCs onto biodegradable polyglycolic acid (PGA) scaffolds, we generated engineered vascular tissues presenting effective collagen deposition which were suitable for implantation into an immunodeficient mice model. In conclusion, our xenogeneic-free conditions for generating hiPSC-VSMCs produce cells with the comparable capacity for vascular tissue engineering as standard xenogeneic protocols, thereby moving the hiPSC-TEVG technology one step closer to safe and efficacious clinical translation.
Keywords: Human induced pluripotent stem cells; vascular smooth muscle cells; vascular tissue engineering; xenogeneic-free.
Copyright © 2020 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Efficient Differentiation of Human Induced Pluripotent Stem Cells into Endothelial Cells under Xenogeneic-free Conditions for Vascular Tissue Engineering.Acta Biomater. 2021 Jan 1;119:184-196. doi: 10.1016/j.actbio.2020.11.007. Epub 2020 Nov 6. Acta Biomater. 2021. PMID: 33166710 Free PMC article.
-
Dynamic three dimensional environment for efficient and large scale generation of smooth muscle cells from hiPSCs.Stem Cell Res Ther. 2024 Dec 3;15(1):463. doi: 10.1186/s13287-024-04053-z. Stem Cell Res Ther. 2024. PMID: 39627821 Free PMC article.
-
Methods for Differentiating hiPSCs into Vascular Smooth Muscle Cells.Methods Mol Biol. 2022;2375:21-34. doi: 10.1007/978-1-0716-1708-3_3. Methods Mol Biol. 2022. PMID: 34591296
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
-
Stem cell insights into human trophoblast lineage differentiation.Hum Reprod Update. 2016 Dec;23(1):77-103. doi: 10.1093/humupd/dmw026. Epub 2016 Sep 2. Hum Reprod Update. 2016. PMID: 27591247
Cited by
-
Regenerative Engineering: Current Applications and Future Perspectives.Front Surg. 2021 Nov 3;8:731031. doi: 10.3389/fsurg.2021.731031. eCollection 2021. Front Surg. 2021. PMID: 34805257 Free PMC article. Review.
-
Bioprocessing Considerations towards the Manufacturing of Therapeutic Skeletal and Smooth Muscle Cells.Bioengineering (Basel). 2023 Sep 9;10(9):1067. doi: 10.3390/bioengineering10091067. Bioengineering (Basel). 2023. PMID: 37760170 Free PMC article. Review.
-
Extracellular vesicles produced by human-induced pluripotent stem cell-derived endothelial cells can prevent arterial stenosis in mice via autophagy regulation.Front Cardiovasc Med. 2022 Oct 17;9:922790. doi: 10.3389/fcvm.2022.922790. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36324745 Free PMC article.
-
ReMeDy: a platform for integrating and sharing published stem cell research data with a focus on iPSC trials.Database (Oxford). 2021 Jun 22;2021:baab038. doi: 10.1093/database/baab038. Database (Oxford). 2021. PMID: 34156448 Free PMC article.
-
Human Induced Pluripotent Stem Cell-Derived Vascular Cells: Recent Progress and Future Directions.J Cardiovasc Dev Dis. 2021 Nov 4;8(11):148. doi: 10.3390/jcdd8110148. J Cardiovasc Dev Dis. 2021. PMID: 34821701 Free PMC article. Review.
References
-
- Feliciano DV, Moore EE, West MA, Moore FA, Davis JW, Cocanour CS, Scalea TM, McIntyre RC Jr., Western Trauma Association critical decisions in trauma: evaluation and management of peripheral vascular injury, part II, The journal of trauma and acute care surgery 75(3) (2013) 391–7. - PubMed
-
- Schild AF, Perez E, Gillaspie E, Seaver C, Livingstone J, Thibonnier A, Arteriovenous fistulae vs. arteriovenous grafts: a retrospective review of 1,700 consecutive vascular access cases, J Vasc Access 9(4) (2008) 231–5. - PubMed
-
- Shum-Tim D, Stock U, Hrkach J, Shinoka T, Lien J, Moses MA, Stamp A, Taylor G, Moran AM, Landis W, Langer R, Vacanti JP, Mayer JE Jr., Tissue engineering of autologous aorta using a new biodegradable polymer, Ann Thorac Surg 68(6) (1999) 2298–304; discussion 2305. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

