Potassium content diminishes in infected cells of Medicago truncatula nodules due to the mislocation of channels MtAKT1 and MtSKOR/GORK
- PMID: 33130893
- PMCID: PMC7904148
- DOI: 10.1093/jxb/eraa508
Potassium content diminishes in infected cells of Medicago truncatula nodules due to the mislocation of channels MtAKT1 and MtSKOR/GORK
Abstract
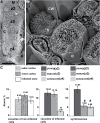
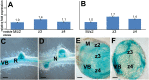
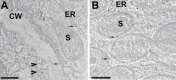
Rhizobia establish a symbiotic relationship with legumes that results in the formation of root nodules, where bacteria encapsulated by a membrane of plant origin (symbiosomes), convert atmospheric nitrogen into ammonia. Nodules are more sensitive to ionic stresses than the host plant itself. We hypothesize that such a high vulnerability might be due to defects in ion balance in the infected tissue. Low temperature SEM (LTSEM) and X-ray microanalysis of Medicago truncatula nodules revealed a potassium (K+) decrease in symbiosomes and vacuoles during the life span of infected cells. To clarify K+ homeostasis in the nodule, we performed phylogenetic and gene expression analyses, and confocal and electron microscopy localization of two key plant Shaker K+ channels, AKT1 and SKOR/GORK. Phylogenetic analyses showed that the genome of some legume species, including the Medicago genus, contained one SKOR/GORK and one AKT1 gene copy, while other species contained more than one copy of each gene. Localization studies revealed mistargeting and partial depletion of both channels from the plasma membrane of M. truncatula mature nodule-infected cells that might compromise ion transport. We propose that root nodule-infected cells have defects in K+ balance due to mislocation of some plant ion channels, as compared with non-infected cells. The putative consequences are discussed.
Keywords: Medicago truncatula; AKT1; GORK; SKOR; X-ray microanalysis; potassium channels; symbiosis; symbiosome.
Figures


References
-
- Adem GD, Chen G, Shabala L, Chen ZH, Shabala S. 2020. GORK channel: a master switch of plant metabolism? Trends in Plant Science 25, 434–445. - PubMed
-
- Andrio E, Marino D, Marmeys A, de Segonzac MD, Damiani I, Genre A, Huguet S, Frendo P, Puppo A, Pauly N. 2013. Hydrogen peroxide-regulated genes in the Medicago truncatula–Sinorhizobium meliloti symbiosis. New Phytologist 198, 179–189. - PubMed
-
- Bertioli DJ, Jenkins J, Clevenger J, et al. 2019. The genome sequence of segmental allotetraploid peanut Arachis hypogaea. Nature Genetics 51, 877–884. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

