Optimal therapeutic targeting by HDAC inhibition in biopsy-derived treatment-naïve diffuse midline glioma models
- PMID: 33130903
- PMCID: PMC7992886
- DOI: 10.1093/neuonc/noaa249
Optimal therapeutic targeting by HDAC inhibition in biopsy-derived treatment-naïve diffuse midline glioma models
Abstract
Background: Diffuse midline gliomas (DMGs), including diffuse intrinsic pontine gliomas (DIPGs), have a dismal prognosis, with less than 2% surviving 5 years postdiagnosis. The majority of DIPGs and all DMGs harbor mutations altering the epigenetic regulatory histone tail (H3 K27M). Investigations addressing DMG epigenetics have identified a few promising drugs, including the HDAC inhibitor (HDACi) panobinostat. Here, we use clinically relevant DMG models to identify and validate other effective HDACi and their biomarkers of response.
Methods: HDAC inhibitors were tested across biopsy-derived treatment-naïve in vitro and in vivo DMG models with biologically relevant radiation resistance. RNA sequencing was performed to define and compare drug efficacy and to map predictive biomarkers of response.
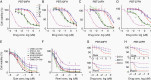
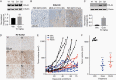
Results: Quisinostat and romidepsin showed efficacy with low nanomolar half-maximal inhibitory concentration (IC50) values (~50 and ~5 nM, respectively). Comparative transcriptome analyses across quisinostat, romidepsin, and panobinostat showed a greater degree of shared biological effects between quisinostat and panobinostat, and less overlap with romidepsin. However, some transcriptional changes were consistent across all 3 drugs at similar biologically effective doses, such as overexpression of troponin T1 slow skeletal type (TNNT1) and downregulation of collagen type 20 alpha 1 chain (COL20A1), identifying these as potential vulnerabilities or on-target biomarkers in DMG. Quisinostat and romidepsin significantly (P < 0.0001) inhibited in vivo tumor growth.
Conclusions: Our data highlight the utility of treatment-naïve biopsy-derived models; establishes quisinostat and romidepsin as effective in vivo; illuminates potential mechanisms and/or biomarkers of DMG cell lethality due to HDAC inhibition; and emphasizes the need for brain tumor-penetrant versions of potentially efficacious agents.
Keywords: H3 K27M-mutant (DMG); diffuse intrinsic pontine glioma (DIPG); diffuse midline glioma; histone deacetylase inhibitor (HDACi); quisinostat; romidepsin.
© The Author(s) 2020. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



Comment in
-
Stemming the growth of pediatric gliomas through histone modification.Neuro Oncol. 2021 Mar 25;23(3):341-342. doi: 10.1093/neuonc/noaa302. Neuro Oncol. 2021. PMID: 33470409 Free PMC article. No abstract available.
References
-
- Barkovich AJ, Krischer J, Kun LE, et al. . Brain stem gliomas: a classification system based on magnetic resonance imaging. Pediatr Neurosurg. 1990;16(2):73–83. - PubMed
-
- Albright AL, Packer RJ, Zimmerman R, Rorke LB, Boyett J, Hammond GD. Magnetic resonance scans should replace biopsies for the diagnosis of diffuse brain stem gliomas: a report from the Children’s Cancer Group. Neurosurgery. 1993;33(6):1026–1029; discussion 1029–1030. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

