Impact of solitary pulmonary nodule size on qualitative and quantitative assessment using 18F-fluorodeoxyglucose PET/CT: the SPUTNIK trial
- PMID: 33130961
- PMCID: PMC8113131
- DOI: 10.1007/s00259-020-05089-y
Impact of solitary pulmonary nodule size on qualitative and quantitative assessment using 18F-fluorodeoxyglucose PET/CT: the SPUTNIK trial
Abstract
Purpose: To compare qualitative and semi-quantitative PET/CT criteria, and the impact of nodule size on the diagnosis of solitary pulmonary nodules in a prospective multicentre trial.
Methods: Patients with an SPN on CT ≥ 8 and ≤ 30 mm were recruited to the SPUTNIK trial at 16 sites accredited by the UK PET Core Lab. Qualitative assessment used a five-point ordinal PET-grade compared to the mediastinal blood pool, and a combined PET/CT grade using the CT features. Semi-quantitative measures included SUVmax of the nodule, and as an uptake ratio to the mediastinal blood pool (SURBLOOD) or liver (SURLIVER). The endpoints were diagnosis of lung cancer via biopsy/histology or completion of 2-year follow-up. Impact of nodule size was analysed by comparison between nodule size tertiles.
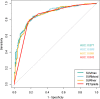
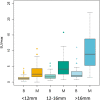
Results: Three hundred fifty-five participants completed PET/CT and 2-year follow-up, with 59% (209/355) malignant nodules. The AUCs of the three techniques were SUVmax 0.87 (95% CI 0.83;0.91); SURBLOOD 0.87 (95% CI 0.83; 0.91, p = 0.30 versus SUVmax); and SURLIVER 0.87 (95% CI 0.83; 0.91, p = 0.09 vs. SUVmax). The AUCs for all techniques remained stable across size tertiles (p > 0.1 for difference), although the optimal diagnostic threshold varied by size. For nodules < 12 mm, an SUVmax of 1.75 or visual uptake equal to the mediastinum yielded the highest accuracy. For nodules > 16 mm, an SUVmax ≥ 3.6 or visual PET uptake greater than the mediastinum was the most accurate.
Conclusion: In this multicentre trial, SUVmax was the most accurate technique for the diagnosis of solitary pulmonary nodules. Diagnostic thresholds should be altered according to nodule size.
Trial registration: ISRCTN - ISRCTN30784948. ClinicalTrials.gov - NCT02013063.
Keywords: Cost-effectiveness; DCE-CT; Diagnostic accuracy trial; Diagnostic imaging; Lung cancer; PET/CT; Solitary pulmonary nodule (SPN).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Horeweg N, van Rosmalen J, Heuvelmans MA, van der Aalst CM, Vliegenthart R, Scholten ET, et al. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol. Elsevier Ltd; 2014;15:1332–41. Available from: 10.1016/S1470-2045(14)70389-4. - PubMed
-
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. http://www.nejm.org/doi/10.1056/NEJMoa1511939. - DOI - PMC - PubMed
-
- Basso Dias A, Zanon M, Altmayer S, Sartori Pacini G, Henz Concatto N, Watte G, et al. Fluorine 18-FDG PET/CT and diffusion-weighted MRI for malignant versus benign pulmonary lesions: a meta-analysis. Radiology. 2019;290:525–34. http://pubs.rsna.org/doi/10.1148/radiol.2018181159. - DOI - PubMed
-
- Li Z-Z, Huang Y-L, Song H-J, Wang Y-J, Huang Y. The value of 18 F-FDG-PET/CT in the diagnosis of solitary pulmonary nodules: a meta-analysis. Med (Baltimore). 2018;97:e0130. Available from: http://journals.lww.com/00005792-201803230-00007. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

