The role of RAGE in host pathology and crosstalk between RAGE and TLR4 in innate immune signal transduction pathways
- PMID: 33131091
- PMCID: PMC8121140
- DOI: 10.1096/fj.202002136R
The role of RAGE in host pathology and crosstalk between RAGE and TLR4 in innate immune signal transduction pathways
Abstract
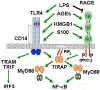
Although the innate immune receptor protein, Receptor for Advanced Glycation End products (RAGE), has been extensively studied, there has been renewed interest in RAGE for its potential role in sepsis, along with a host of other inflammatory diseases of chronic, noninfectious origin. In contrast to other innate immune receptors, for example, Toll-like receptors (TLRs), that recognize ligands derived from pathogenic organisms that are collectively known as "pathogen-associated molecular patterns" (PAMPs) or host-derived "damage-associated molecular patterns" (DAMPs), RAGE has been shown to recognize a broad collection of DAMPs exclusively. Historically, these DAMPs have been shown to be pro-inflammatory in nature. Early studies indicated that the adaptor molecule, MyD88, might be important for this change. More recent studies have explored further the mechanisms underlying this inflammatory change. Overall, the newer results have shown that there is extensive crosstalk between RAGE and TLRs. The three canonical RAGE ligands, Advanced Glycation End products (AGEs), HMGB1, and S100 proteins, have all been shown to activate both TLRs and RAGE to varying degrees in order to induce inflammation in in vitro models. As with any field that delves deeply into innate signaling, obstacles of reagent purity may be a cause of some of the discrepancies in the literature, and we have found that commercial antibodies that have been widely used exhibit a high degree of nonspecificity. Nonetheless, the weight of published evidence has led us to speculate that RAGE may be physically interacting with TLRs on the cell surface to elicit inflammation via MyD88-dependent signaling.
Keywords: DAMPs; HMGB1; inflammation; toll-like receptors.
© 2020 Federation of American Societies for Experimental Biology.
Conflict of interest statement
Conflict of Interest Disclosure:
The authors declare no conflict of interest. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Institute of Allergy and Infectious Diseases.
Figures
Similar articles
-
RAGE and TLRs: relatives, friends or neighbours?Mol Immunol. 2013 Dec;56(4):739-44. doi: 10.1016/j.molimm.2013.07.008. Epub 2013 Aug 14. Mol Immunol. 2013. PMID: 23954397 Review.
-
Targeting RAGE Signaling in Inflammatory Disease.Annu Rev Med. 2018 Jan 29;69:349-364. doi: 10.1146/annurev-med-041316-085215. Epub 2017 Nov 6. Annu Rev Med. 2018. PMID: 29106804 Review.
-
Interplay between RAGE and TLR4 Regulates HMGB1-Induced Inflammation by Promoting Cell Surface Expression of RAGE and TLR4.J Immunol. 2020 Aug 1;205(3):767-775. doi: 10.4049/jimmunol.1900860. Epub 2020 Jun 24. J Immunol. 2020. PMID: 32580932
-
Insight into the mechanisms regulating immune homeostasis in health and disease.Asian Pac J Allergy Immunol. 2011 Mar;29(1):1-14. Asian Pac J Allergy Immunol. 2011. PMID: 21560483 Review.
-
RAGE and TLRs as Key Targets for Antiatherosclerotic Therapy.Biomed Res Int. 2018 Aug 26;2018:7675286. doi: 10.1155/2018/7675286. eCollection 2018. Biomed Res Int. 2018. PMID: 30225265 Free PMC article. Review.
Cited by
-
Inhibitory pattern recognition receptors.J Exp Med. 2022 Jan 3;219(1):e20211463. doi: 10.1084/jem.20211463. Epub 2021 Dec 14. J Exp Med. 2022. PMID: 34905019 Free PMC article. Review.
-
AGEs-RAGE-KCa3.1 pathway mediates palmitic acid-induced migration of PBMCs from patients with type 2 diabetes.Heliyon. 2023 Mar 24;9(4):e14823. doi: 10.1016/j.heliyon.2023.e14823. eCollection 2023 Apr. Heliyon. 2023. PMID: 37025887 Free PMC article.
-
Mapping the transcriptomic changes of endothelial compartment in human hippocampus across aging and mild cognitive impairment.Biol Open. 2021 May 15;10(5):bio057950. doi: 10.1242/bio.057950. Epub 2021 May 17. Biol Open. 2021. PMID: 34184731 Free PMC article.
-
RAGE-TLR4 Crosstalk Is the Key Mechanism by Which High Glucose Enhances the Lipopolysaccharide-Induced Inflammatory Response in Primary Bovine Alveolar Macrophages.Int J Mol Sci. 2023 Apr 10;24(8):7007. doi: 10.3390/ijms24087007. Int J Mol Sci. 2023. PMID: 37108174 Free PMC article.
-
Involvement of RAGE and Oxidative Stress in Inflammatory and Infectious Skin Diseases.Antioxidants (Basel). 2021 Jan 9;10(1):82. doi: 10.3390/antiox10010082. Antioxidants (Basel). 2021. PMID: 33435332 Free PMC article. Review.
References
-
- Neeper M, Schmidt AM, Brett J, et al. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem 1992;267(21):14998–5004. - PubMed
-
- Sugaya K, Fukagawa T, Matsumoto K, et al. Three genes in the human MHC class III region near the junction with the class II: gene for receptor of advanced glycosylation end products, PBX2 homeobox gene and a notch homolog, human counterpart of mouse mammary tumor gene int-3. Genomics 1994;23(2):408–19. - PubMed
-
- Schlueter C, Hauke S, Flohr AM, Rogalla P, Bullerdiek J. Tissue-specific expression patterns of the RAGE receptor and its soluble forms--a result of regulated alternative splicing? Biochim Biophys Acta 2003;1630(1):1–6. - PubMed
-
- Hudson BI, Carter AM, Harja E, et al. Identification, classification, and expression of RAGE gene splice variants. FASEB J 2008;22(5):1572–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

