Diagnostic Salivary Tests for SARS-CoV-2
- PMID: 33131360
- PMCID: PMC7604673
- DOI: 10.1177/0022034520969670
Diagnostic Salivary Tests for SARS-CoV-2
Abstract
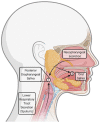
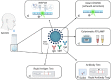
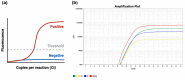
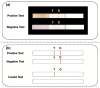
The diagnosis of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection relies on the detection of viral RNA by real-time reverse transcription polymerase chain reaction (rRT-PCR) performed with respiratory specimens, especially nasopharyngeal swabs. However, this procedure requires specialized medical personnel, centralized laboratory facilities, and time to provide results (from several hours up to 1 d). In addition, there is a non-negligible risk of viral transmission for the operator who performs the procedure. For these reasons, several studies have suggested the use of other body fluids, including saliva, for the detection of SARS-CoV-2. The use of saliva as a diagnostic specimen has numerous advantages: it is easily self-collected by the patient with almost no discomfort, it does not require specialized health care personnel for its management, and it reduces the risks for the operator. In the past few months, several scientific papers, media, and companies have announced the development of new salivary tests to detect SARS-CoV-2 infection. Posterior oropharyngeal saliva should be distinguished from oral saliva, since the former is a part of respiratory secretions, while the latter is produced by the salivary glands, which are outside the respiratory tract. Saliva can be analyzed through standard (rRT-PCR) or rapid molecular biology tests (direct rRT-PCR without extraction), although, in a hospital setting, these procedures may be performed only in addition to nasopharyngeal swabs to minimize the incidence of false-negative results. Conversely, the promising role of saliva in the diagnosis of SARS-CoV-2 infection is highlighted by the emergence of point-of-care technologies and, most important, point-of-need devices. Indeed, these devices can be directly used in workplaces, airports, schools, cinemas, and shopping centers. An example is the recently described Rapid Salivary Test, an antigen test based on the lateral flow assay, which detects the presence of the virus by identifying the spike protein in the saliva within a few minutes.
Keywords: COVID-19; SARS-CoV-2; Severe acute respiratory syndrome-related coronavirus; coronavirus; point-of-care testing; saliva.
Conflict of interest statement
Figures

Similar articles
-
Diagnostic Performance of Self-Collected Saliva Versus Nasopharyngeal Swab for the Molecular Detection of SARS-CoV-2 in the Clinical Setting.Microbiol Spectr. 2021 Dec 22;9(3):e0046821. doi: 10.1128/Spectrum.00468-21. Epub 2021 Nov 3. Microbiol Spectr. 2021. PMID: 34730436 Free PMC article.
-
COVID-19 screening test by using random oropharyngeal saliva.J Med Virol. 2021 Apr;93(4):2461-2466. doi: 10.1002/jmv.26773. Epub 2021 Jan 22. J Med Virol. 2021. PMID: 33393672 Free PMC article.
-
Saliva samples for detection of SARS-CoV-2 in mildly symptomatic and asymptomatic patients.J Med Virol. 2021 May;93(5):2932-2937. doi: 10.1002/jmv.26821. Epub 2021 Feb 9. J Med Virol. 2021. PMID: 33501645 Free PMC article.
-
Detection profile of SARS-CoV-2 using RT-PCR in different types of clinical specimens: A systematic review and meta-analysis.J Med Virol. 2021 Feb;93(2):719-725. doi: 10.1002/jmv.26349. Epub 2020 Aug 2. J Med Virol. 2021. PMID: 32706393 Free PMC article.
-
Saliva as a possible tool for the SARS-CoV-2 detection: A review.Travel Med Infect Dis. 2020 Nov-Dec;38:101920. doi: 10.1016/j.tmaid.2020.101920. Epub 2020 Nov 19. Travel Med Infect Dis. 2020. PMID: 33220456 Free PMC article. Review.
Cited by
-
Saliva sample for detection of SARS-CoV-2: A possible alternative for mass testing.PLoS One. 2022 Sep 28;17(9):e0275201. doi: 10.1371/journal.pone.0275201. eCollection 2022. PLoS One. 2022. PMID: 36170269 Free PMC article.
-
A Multiplex One-Step RT-qPCR Protocol to Detect SARS-CoV-2 in NP/OP Swabs and Saliva.Curr Protoc. 2021 May;1(5):e145. doi: 10.1002/cpz1.145. Curr Protoc. 2021. PMID: 34004070 Free PMC article.
-
Development of practical techniques for simultaneous detection and distinction of current and emerging SARS-CoV-2 variants.Anal Sci. 2023 Nov;39(11):1839-1856. doi: 10.1007/s44211-023-00396-4. Epub 2023 Jul 30. Anal Sci. 2023. PMID: 37517003 Review.
-
Role of Laboratory Medicine in SARS-CoV-2 Diagnostics. Lessons Learned from a Pandemic.Healthcare (Basel). 2021 Jul 19;9(7):915. doi: 10.3390/healthcare9070915. Healthcare (Basel). 2021. PMID: 34356292 Free PMC article. Review.
-
Infection of the oral cavity with SARS-CoV-2 variants: Scope of salivary diagnostics.Front Oral Health. 2022 Oct 31;3:1001790. doi: 10.3389/froh.2022.1001790. eCollection 2022. Front Oral Health. 2022. PMID: 36389278 Free PMC article. Review.
References
-
- Adhikari U, Chabrelie A, Weir M, Boehnke K, McKenzie E, Ikner L, Wang M, Wang Q, Young K, Haas CN, et al. 2019. A case study evaluating the risk of infection from Middle Eastern respiratory syndrome coronavirus (MERS-CoV) in a hospital setting through bioaerosols. Risk Anal. 39(12):2608–2624. - PMC - PubMed
-
- Becker D, Sandoval E, Amin A. 2020. Saliva is less sensitive than nasopharyngeal swabs for COVID-19 detection in the community setting. medRxiv (preprint). doi: 10.1101/2020.05.11.20092338 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

