Depletion of circ_0007841 inhibits multiple myeloma development and BTZ resistance via miR-129-5p/JAG1 axis
- PMID: 33131409
- PMCID: PMC7751678
- DOI: 10.1080/15384101.2020.1839701
Depletion of circ_0007841 inhibits multiple myeloma development and BTZ resistance via miR-129-5p/JAG1 axis
Abstract
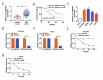
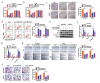
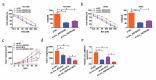
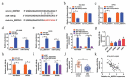
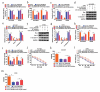
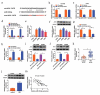
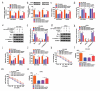
Circular RNAs (circRNAs) possess important regulatory effects on multiple myeloma (MM) progression. Here, we aimed at exploring the function of circ_0007841 in MM and the underlying molecular mechanism. Expression of circ_0007841, microRNA (miR)-129-5p and Jagged1 (JAG1) was determined via qRT-PCR or western blot assay. Methyl thiazolyl tetrazolium (MTT) assay was applied to examine cell viability and IC50 value of MM cells to bortezomib (BTZ). Colony formation assay was performed to analyze cell proliferation. Moreover, cell apoptosis was assessed by flow cytometry and western blot analysis. Cell metastasis was evaluated by wound healing assay and Transwell assay. Function of circ_0007841 in vivo was determined by xenograft tumor assay. Target relationship between miR-129-5p and circ_0007841 or JAG1 was confirmed via dual-luciferase reporter, RNA immunoprecipitation (RIP) and pull-down assays. The up-regulation of circ_0007841 and JAG1, and the down-regulation of miR-129-5p were detected in MM bone marrow aspirates and cells. Circ_0007841 knockdown significantly repressed cell proliferation, chemoresistance, and metastasis, while contributed to apoptosis of MM cells in vitro, and reduced tumor growth in vivo. Circ_0007841 targeted miR-129-5p, and miR-129-5p inhibition reversed impact of silencing of circ_0007841 on MM cells. JAG1 was a mRNA target of miR-129-5p, whose overexpression could undermine the miR-129-5p-mediated effects on MM cells. Circ_0007841 positively regulated JAG1 expression via absorbing miR-129-5p. Circ_0007841 knockdown inhibited MM cell proliferation, metastasis and chemoresistance through modulating miR-129-5p/JAG1 axis, suggesting that circ_0007841 might serve as a potential therapeutic target of MM.
Keywords: JAG1; Multiple myeloma; chemoresistance; circ_0007841; metastasis; miR-129-5p; proliferation.
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures
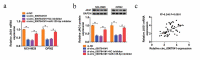
Similar articles
-
Novel insights into the circ_0003489/let-7b-5p/GLUT1 axis and its possible role in multiple myeloma.Transpl Immunol. 2025 Feb;88:102165. doi: 10.1016/j.trim.2024.102165. Epub 2024 Dec 21. Transpl Immunol. 2025. PMID: 39716648
-
Circ_PIP5K1A regulates cisplatin resistance and malignant progression in non-small cell lung cancer cells and xenograft murine model via depending on miR-493-5p/ROCK1 axis.Respir Res. 2021 Sep 18;22(1):248. doi: 10.1186/s12931-021-01840-7. Respir Res. 2021. PMID: 34537072 Free PMC article.
-
Circ_0005615 contributes to the progression and Bortezomib resistance of multiple myeloma by sponging miR-185-5p and upregulating IRF4.Anticancer Drugs. 2022 Oct 1;33(9):893-902. doi: 10.1097/CAD.0000000000001378. Epub 2022 Sep 14. Anticancer Drugs. 2022. PMID: 36136989
-
Inhibition of hsa_circ_0003489 shifts balance from autophagy to apoptosis and sensitizes multiple myeloma cells to bortezomib via miR-874-3p/HDAC1 axis.J Gene Med. 2021 Sep;23(9):e3329. doi: 10.1002/jgm.3329. Epub 2021 Jun 30. J Gene Med. 2021. PMID: 33625798
-
Identification of MicroRNAs With In Vivo Efficacy in Multiple Myeloma-related Xenograft Models.Cancer Genomics Proteomics. 2020 Jul-Aug;17(4):321-334. doi: 10.21873/cgp.20192. Cancer Genomics Proteomics. 2020. PMID: 32576578 Free PMC article. Review.
Cited by
-
Identifying Hub Genes Associated with Neoadjuvant Chemotherapy Resistance in Breast Cancer and Potential Drug Repurposing for the Development of Precision Medicine.Int J Mol Sci. 2022 Oct 20;23(20):12628. doi: 10.3390/ijms232012628. Int J Mol Sci. 2022. PMID: 36293493 Free PMC article.
-
CircRNAs: novel therapeutic targets in multiple myeloma.Mol Biol Rep. 2022 Nov;49(11):10667-10676. doi: 10.1007/s11033-022-07668-8. Epub 2022 Jun 21. Mol Biol Rep. 2022. PMID: 35729478 Review.
-
Epigenetic Alterations as Vital Aspects of Bortezomib Molecular Action.Cancers (Basel). 2023 Dec 23;16(1):84. doi: 10.3390/cancers16010084. Cancers (Basel). 2023. PMID: 38201512 Free PMC article. Review.
-
Hsa_Circ_0134426 Attenuates the Malignant Biological Behaviors of Multiple Myeloma by Suppressing miR-146b-3p to Upregulate NDNF.Mol Biotechnol. 2023 Jul;65(7):1165-1177. doi: 10.1007/s12033-022-00618-6. Epub 2022 Dec 2. Mol Biotechnol. 2023. PMID: 36460812
-
Circ_0138960 knockdown alleviates lipopolysaccharide-induced inflammatory response and injury in human dental pulp cells by targeting miR-545-5p/MYD88 axis in pulpitis.J Dent Sci. 2023 Jan;18(1):191-202. doi: 10.1016/j.jds.2022.06.012. Epub 2022 Jul 2. J Dent Sci. 2023. PMID: 36643232 Free PMC article.
References
-
- Palumbo A, Anderson K.. Multiple myeloma. N Engl J Med. 2011;364(11):1046–1060. - PubMed
-
- Vrabel D, Pour L, Sevcikova S.. The impact of NF-kappaB signaling on pathogenesis and current treatment strategies in multiple myeloma. Blood Rev. 2019;34:56–66. - PubMed
-
- Dimopoulos MA, Terpos E. Multiple myeloma. Ann Oncol. 2010;21(Suppl 7):vii143–150. - PubMed
-
- Kehrer M, Koob S, Kehrer A, et al. Multiple Myeloma - Current Standards in Surgical Treatment. Z Orthop Unfall. 2019;157:164–172. - PubMed
-
- Landgren O, Iskander K. Modern multiple myeloma therapy: deep, sustained treatment response and good clinical outcomes. J Intern Med. 2017;281:365–382. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
