Improving low-temperature activity and thermostability of exo-inulinase InuAGN25 on the basis of increasing rigidity of the terminus and flexibility of the catalytic domain
- PMID: 33131413
- PMCID: PMC8291790
- DOI: 10.1080/21655979.2020.1837476
Improving low-temperature activity and thermostability of exo-inulinase InuAGN25 on the basis of increasing rigidity of the terminus and flexibility of the catalytic domain
Abstract
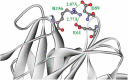
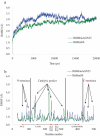
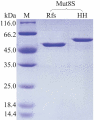
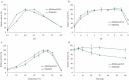
Enzymes displaying high activity at low temperatures and good thermostability are attracting attention in many studies. However, improving low-temperature activity along with the thermostability of enzymes remains challenging. In this study, the mutant Mut8S, including eight sites (N61E, K156R, P236E, T243K, D268E, T277D, Q390K, and R409D) mutated from the exo-inulinase InuAGN25, was designed on the basis of increasing the number of salt bridges through comparison between the low-temperature-active InuAGN25 and thermophilic exo-inulinases. The recombinant Mut8S, which was expressed in Escherichia coli, was digested by human rhinovirus 3 C protease to remove the amino acid fusion sequence at N-terminus, producing RfsMut8S. Compared with wild-type RfsMInuAGN25, the mutant RfsMut8S showed (1) lower root mean square deviation values, (2) lower root mean square fluctuation (RMSF) values of residues in six regions of the N and C termini but higher RMSF values in five regions of the catalytic pocket, (3) higher activity at 0-40°C, and (4) better thermostability at 50°C. This study proposes a way to increase low-temperature activity along with a thermostability improvement of exo-inulinase on the basis of increasing the rigidity of the terminus and the flexibility of the catalytic domain. These findings may prove useful in formulating rational designs for increasing the thermal performance of enzymes.
Keywords: Enzyme; biochemical property; mechanism; mutagenesis; structure.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Removal of N-terminal tail changes the thermostability of the low-temperature-active exo-inulinase InuAGN25.Bioengineered. 2020 Dec;11(1):921-931. doi: 10.1080/21655979.2020.1809921. Bioengineered. 2020. PMID: 32865156 Free PMC article.
-
Deletion of loop fragment adjacent to active site diminishes the stability and activity of exo-inulinase.Int J Biol Macromol. 2016 Nov;92:1234-1241. doi: 10.1016/j.ijbiomac.2016.08.039. Epub 2016 Aug 12. Int J Biol Macromol. 2016. PMID: 27527695
-
Improvement of thermostability by increasing rigidity in the finger regions and flexibility in the catalytic pocket area of Pseudoalteromonas porphyrae κ-carrageenase.World J Microbiol Biotechnol. 2024 May 28;40(7):216. doi: 10.1007/s11274-024-04029-4. World J Microbiol Biotechnol. 2024. PMID: 38802708
-
Molecular characterization and expression of microbial inulinase genes.Crit Rev Microbiol. 2013 May;39(2):152-65. doi: 10.3109/1040841X.2012.694411. Epub 2012 Jun 26. Crit Rev Microbiol. 2013. PMID: 22734928 Review.
-
Rational protein design for thermostabilization of glycoside hydrolases based on structural analysis.Appl Microbiol Biotechnol. 2018 Oct;102(20):8677-8684. doi: 10.1007/s00253-018-9288-7. Epub 2018 Aug 14. Appl Microbiol Biotechnol. 2018. PMID: 30109396 Review.
Cited by
-
The unique salt bridge network in GlacPETase: a key to its stability.Appl Environ Microbiol. 2024 Mar 20;90(3):e0224223. doi: 10.1128/aem.02242-23. Epub 2024 Feb 15. Appl Environ Microbiol. 2024. PMID: 38358247 Free PMC article.
-
Deletion of the Loop Linking Two Domains of Exo-Inulinase InuAMN8 Diminished the Enzymatic Thermo-Halo-Alcohol Tolerance.Front Microbiol. 2022 Jun 23;13:924447. doi: 10.3389/fmicb.2022.924447. eCollection 2022. Front Microbiol. 2022. PMID: 35814689 Free PMC article.
References
-
- Singh RS, Singh T, Larroche C.. Biotechnological applications of inulin-rich feedstocks. Bioresour Technol. 2019;273:641–653. - PubMed
-
- Singh PK, Kumar V, Yadav R, et al. Bioengineering for microbial inulinases: trends and applications. Current Protein Pept Sci. 2017;18:966–972. - PubMed
-
- Pucci F, Rooman M. Physical and molecular bases of protein thermal stability and cold adaptation. Curr Opin Struct Biol. 2017;42:117–128. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
