Universal access to essential medicines as part of the right to health: a cross-national comparison of national laws, medicines policies, and health system indicators
- PMID: 33131456
- PMCID: PMC7605313
- DOI: 10.1080/16549716.2019.1699342
Universal access to essential medicines as part of the right to health: a cross-national comparison of national laws, medicines policies, and health system indicators
Abstract
Background: Access to essential medicines for the world's poor and vulnerable has made little progress since 2000, except for a few specific medicines such as antiretrovirals for HIV/AIDS. Human rights principles written into national law can create a supportive environment for universal access to medicines; however, systematic research and policy guidance on this topic is lacking.
Objective: To examine how international human rights law and WHO's essential medicines policies are embedded in national law for medicines affordability and financing, and interpreted and implemented in practice to promote universal access to essential medicines.
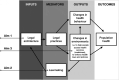
Methods: This thesis consists of (1) a cross-national content analysis of 192 national constitutions, 71 national medicines policies, and legislation for universal health coverage (UHC) from 16 mostly low- and middle-income countries; (2) a case study of medicines litigation in Uruguay, and (3) a follow-up report of eight right to health indicators for access to medicines from 195 countries.
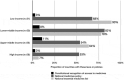
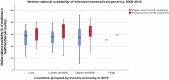
Results: Some, but not all, of the 12 principles from human rights law and WHO's policy are embedded in national UHC law and medicines policies (part 1). Even the most rights-compliant legislation for access to medicines is subject to the unique and inconsistent interpretation of domestic courts, which may be inconsistent with the right to health in international law (part 2). Many national health systems for which data were available still fail to meet the official targets for eight indicators of access to medicines (part 3).
Conclusions: International human rights law and WHO policy are embedded in national law for essential medicines and practically implemented in national health systems. Law makers can use these findings and the example texts in this thesis as a starting point for writing and monitoring governments' rights-based legal commitments for access to medicines. Future research should study the effect of national law on access to medicines and population health.
Keywords: Access to medicines; constitution; essential drugs; health financing; human rights; litigation; national medicines policy; pharmaceutical policy; universal health coverage; vulnerable populations.
Conflict of interest statement
No potential conflict of interest was reported by the author.
Figures
Similar articles
-
Legislating for universal access to medicines: a rights-based cross-national comparison of UHC laws in 16 countries.Health Policy Plan. 2019 Dec 1;34(Supplement_3):iii48-iii57. doi: 10.1093/heapol/czy101. Health Policy Plan. 2019. PMID: 31816073 Free PMC article.
-
The right to health as the basis for universal health coverage: A cross-national analysis of national medicines policies of 71 countries.PLoS One. 2019 Jun 28;14(6):e0215577. doi: 10.1371/journal.pone.0215577. eCollection 2019. PLoS One. 2019. PMID: 31251737 Free PMC article.
-
Essential Medicines in Universal Health Coverage: A Scoping Review of Public Health Law Interventions and How They Are Measured in Five Middle-Income Countries.Int J Environ Res Public Health. 2020 Dec 18;17(24):9524. doi: 10.3390/ijerph17249524. Int J Environ Res Public Health. 2020. PMID: 33353250 Free PMC article.
-
Access to essential medicines in 195 countries: A human rights approach to sustainable development.Glob Public Health. 2019 Mar;14(3):431-444. doi: 10.1080/17441692.2018.1515237. Epub 2018 Sep 6. Glob Public Health. 2019. PMID: 30187828
-
Driving a decade of change: HIV/AIDS, patents and access to medicines for all.J Int AIDS Soc. 2011 Mar 27;14:15. doi: 10.1186/1758-2652-14-15. J Int AIDS Soc. 2011. PMID: 21439089 Free PMC article. Review.
Cited by
-
"...In Kiambu county, there are different things being done": A qualitative exploration of healthcare workers' experiences with cord care practices.PLoS One. 2025 Jun 18;20(6):e0326506. doi: 10.1371/journal.pone.0326506. eCollection 2025. PLoS One. 2025. PMID: 40532030 Free PMC article.
-
Anesthetic drug shortages in Pakistan: a multicentre nationwide survey.Can J Anaesth. 2023 Mar;70(3):335-342. doi: 10.1007/s12630-022-02381-3. Epub 2022 Dec 28. Can J Anaesth. 2023. PMID: 36577892 Free PMC article.
-
Prices and Affordability of Essential Medicines in 72 Low-, Middle-, and High-Income Markets.JAMA Health Forum. 2025 Aug 1;6(8):e252043. doi: 10.1001/jamahealthforum.2025.2043. JAMA Health Forum. 2025. PMID: 40815523 Free PMC article.
-
How and why pharmaceutical reforms contribute to universal health coverage through improving equitable access to medicines: a case of Ghana.Front Public Health. 2023 Jul 7;11:1163342. doi: 10.3389/fpubh.2023.1163342. eCollection 2023. Front Public Health. 2023. PMID: 37483923 Free PMC article.
-
The viewpoints of medical sciences wealth creators regarding the wealth creation strategies and path in medical sciences universities.J Educ Health Promot. 2022 Apr 28;11:131. doi: 10.4103/jehp.jehp_978_21. eCollection 2022. J Educ Health Promot. 2022. PMID: 35677280 Free PMC article.
References
-
- World Health Organization, World Bank . Tracking universal health coverage: first global monitoring report [Internet]. Geneva; 2015. [cited 2019 February13]. Available from: https://apps.who.int/iris/bitstream/handle/10665/174536/9789241564977_en...
-
- Wirtz VJ, Hogerzeil HV, Gray AL, et al. Essential medicines for universal health coverage. Lancet [Internet]. 2016. cited 2018 May18;74. Available from: http://apps.who.int/medicinedocs/documents/s23079en/s23079en.pdf - PubMed
-
- WHO Expert Committee on the Selection and Use of Essential Medicines . The selection and use of essential medicines [WHO technical report series, No. 914] [Internet]. Geneva: World Health Organization; 2003. cited 2018 May18. Available from: http://apps.who.int/medicinedocs/en/d/Js4875e/ - PubMed
-
- Mahmić-Kaknjo M, Jeličić-Kadić A, Utrobičić A, et al. Essential medicines availability is still suboptimal in many countries: a scoping review. J Clin Epidemiol [Internet]. 2018. June 1 cited 2018 May24;98:41–16. Available from: https://www.sciencedirect.com/science/article/pii/S0895435617310089 - PubMed
-
- Khatib R, McKee M, Shannon H, et al. Availability and affordability of cardiovascular disease medicines and their effect on use in high-income, middle-income, and low-income countries: an analysis of the PURE study data. Lancet [Internet]. 2016. January 2 cited 2019 February13;387:61–69. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673615004699 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
