Mechanisms of SARS-CoV-2 Transmission and Pathogenesis
- PMID: 33132005
- PMCID: PMC7556779
- DOI: 10.1016/j.it.2020.10.004
Mechanisms of SARS-CoV-2 Transmission and Pathogenesis
Abstract
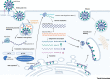
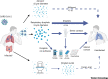
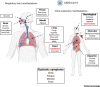
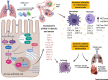
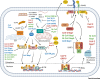
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) marks the third highly pathogenic coronavirus to spill over into the human population. SARS-CoV-2 is highly transmissible with a broad tissue tropism that is likely perpetuating the pandemic. However, important questions remain regarding its transmissibility and pathogenesis. In this review, we summarize current SARS-CoV-2 research, with an emphasis on transmission, tissue tropism, viral pathogenesis, and immune antagonism. We further present advances in animal models that are important for understanding the pathogenesis of SARS-CoV-2, vaccine development, and therapeutic testing. When necessary, comparisons are made from studies with SARS to provide further perspectives on coronavirus infectious disease 2019 (COVID-19), as well as draw inferences for future investigations.
Keywords: COVID-19; SARS, SARS-CoV-2; coronavirus; severe acute respiratory syndrome.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
References
-
- WHO . WHO; 2020. Coronavirus Disease (COVID-19) Pandemic.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

