In vitro growth of bovine oocytes in oocyte-cumulus cell complexes and the effect of follicle stimulating hormone on the growth of oocytes
- PMID: 33132227
- PMCID: PMC7902213
- DOI: 10.1262/jrd.2020-102
In vitro growth of bovine oocytes in oocyte-cumulus cell complexes and the effect of follicle stimulating hormone on the growth of oocytes
Abstract
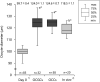
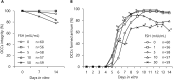
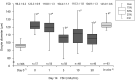
Several successful in vitro culture experiments have used oocyte-cumulus cell-mural granulosa cell complexes (OCGCs) from early antral follicles (0.5-0.7 mm) for the growth of bovine oocytes. However, in studies related to in vitro oocyte maturation and in vitro embryo production, oocyte-cumulus cell complexes (OCCs) that have no mural granulosa cells have been widely used instead of OCGCs. The purpose of this study was to determine whether cumulus cells alone support oocyte growth. First, OCCs and OCGCs were cultured in vitro for 14 days to compare the integrity of the complexes as well as antrum formation. After 14 days, the diameter and meiotic competence of oocytes in OCCs and OCGCs were examined. Oocytes in OCCs grew fully and acquired meiotic competence similar to OCGCs, whereas antrum formation occurred later in OCCs as compared to OCGCs. Subsequently, the effects of follicle stimulating hormone (FSH) on in vitro growth of OCCs were examined for 14 days. When FSH was added to the culture medium, OCCs formed antrum-like structures one day earlier than those cultured without FSH. Oocytes cultured with 1 mIU/ml FSH grew fully and acquired meiotic competence. In contrast, when oocytes were cultured in media containing high concentrations of FSH, some of the OCCs collapsed and the number of degenerated oocytes increased. In conclusion, bovine oocytes in OCCs grow and acquire meiotic competence similar to OCGCs and, 1 mIU/ml FSH supports the development of OCCs and oocyte growth as observed in our culture system.
Keywords: Bovine oocyte; Cumulus cell; Follicle stimulating hormone (FSH); In vitro growth.
Figures



Similar articles
-
Relationship between in vitro growth of bovine oocytes and steroidogenesis of granulosa cells cultured in medium supplemented with bone morphogenetic protein-4 and follicle stimulating hormone.Theriogenology. 2017 Jul 15;97:113-123. doi: 10.1016/j.theriogenology.2017.04.030. Epub 2017 Apr 23. Theriogenology. 2017. PMID: 28583594
-
Effects of oocyte-derived growth factors on the growth of porcine oocytes and oocyte-cumulus cell complexes in vitro.J Reprod Dev. 2021 Aug 27;67(4):273-281. doi: 10.1262/jrd.2021-026. Epub 2021 Jul 14. J Reprod Dev. 2021. PMID: 34261834 Free PMC article.
-
A pre-in vitro maturation medium containing cumulus oocyte complex ligand-receptor signaling molecules maintains meiotic arrest, supports the cumulus oocyte complex and improves oocyte developmental competence.Mol Hum Reprod. 2017 Sep 1;23(9):594-606. doi: 10.1093/molehr/gax032. Mol Hum Reprod. 2017. PMID: 28586460
-
Regulation of cumulus expansion and hyaluronan synthesis in porcine oocyte-cumulus complexes during in vitro maturation.Endocr Regul. 2012 Oct;46(4):225-35. doi: 10.4149/endo_2012_04_225. Endocr Regul. 2012. PMID: 23127506 Review.
-
In vitro growth of immature bovine follicles and oocytes.Reprod Fertil Dev. 2019 Jan;32(2):1-6. doi: 10.1071/RD19270. Reprod Fertil Dev. 2019. PMID: 32188553 Review.
Cited by
-
Effects of total gonadotropin dose on embryo quality and clinical outcomes with AMH stratification in IVF cycles: a retrospective analysis of 12,588 patients.Eur J Med Res. 2024 Mar 12;29(1):167. doi: 10.1186/s40001-024-01768-w. Eur J Med Res. 2024. PMID: 38475829 Free PMC article.
-
Reestablishment of transzonal projections and growth of bovine oocytes in vitro.J Reprod Dev. 2021 Oct 29;67(5):300-306. doi: 10.1262/jrd.2021-036. Epub 2021 Aug 20. J Reprod Dev. 2021. PMID: 34421085 Free PMC article.
-
Change in the ability of bovine granulosa cells to elongate transzonal projections and their transcriptome changes during follicle development.J Reprod Dev. 2024 Dec 13;70(6):362-371. doi: 10.1262/jrd.2024-016. Epub 2024 Oct 14. J Reprod Dev. 2024. PMID: 39401905 Free PMC article.
References
-
- Yamamoto K, Otoi T, Koyama N, Horikita N, Tachikawa S, Miyano T. Development to live young from bovine small oocytes after growth, maturation and fertilization in vitro. Theriogenology 1999; 52: 81–89. - PubMed
-
- Hirao Y, Itoh T, Shimizu M, Iga K, Aoyagi K, Kobayashi M, Kacchi M, Hoshi H, Takenouchi N. In vitro growth and development of bovine oocyte-granulosa cell complexes on the flat substratum: effects of high polyvinylpyrrolidone concentration in culture medium. Biol Reprod 2004; 70: 83–91. - PubMed
-
- Huang W, Kang SS, Nagai K, Yanagawa Y, Takahashi Y, Nagano M. Mitochondrial activity during pre-maturational culture in in vitro-grown bovine oocytes is related to maturational and developmental competences. Reprod Fertil Dev 2016; 28: 349–356. - PubMed
-
- Makita M, Miyano T. Steroid hormones promote bovine oocyte growth and connection with granulosa cells. Theriogenology 2014; 82: 605–612. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

